INTRODUCTION
SDH is of agricultural importance, as many fungi-cides have been and/or will be designed specifical-ly targeting this enzyme in plant fungal pathogens (Moosavi et al. 2020). SDHI fungicides are inhibi-tors of mitochondrial respiration, which act by block-ing the pathogen's succinate dehydrogenase, inter-ruptingelectron flow and respiration (Tian et al. 2020).
Aerobic respiration is a biological process that con-stitutes the main source of energy for living organ-isms, since mitochondrial ATP synthesis depends on the ETC, which couples the generation of an elec-trochemical gradient to the oxidation of NADH. and FADH2 and the reduction of oxygen to water. ETC is composed of four complexes and two mobile electron carriers (coenzyme Q and cytochrome c) (Van Vran-ken et al. 2015).
Three cellular processes are involved in aerobic res-piration: glycolysis, the TCA, and OXPHOS (Moosavi et al. 2020). Electrons derived from the oxidation of NADH by complex I or TCA succinate by the enzyme succinate dehydrogenase, also known as complex II (CII) of ETC or succinate: ubiquinone oxidoreduc-tase (SQR) (Moosavi et al. 2020), are passed along the ETC, along with pumping protons and establish-ing the proton gradient across the inner mitochon-drial membrane. Ultimately, the controlled flow of protons in this electrochemical gradient is utilized by complex V (ATP synthase) to catalyze ATP synthesis (Van Vranken et al. 2015).
The normal enzyme activity of SDH serves to sup-press tumors in humans (Van Vranken et al. 2015). However, mutation that results in loss of function in any of the four subunits (SDHA, SDHB, SDHC, and SDHD), destabilizes the SDH protein complex and eliminates its enzymatic activity (Zhao et al. 2020). SDH expression is mainly regulated through genetic and epigenetic mechanisms, while biochemical factors mainly regulate the activity of this enzyme (Moosavi et al. 2020). The loss or decrease of SDH activity leads to the accumulation of succinate that induces epigen-etic changes in cancer cells (Dalla Pozza et al. 2020). Because of the universal role of SDH in cellular respiration and mitochondrial metabolism in living organ-isms, studies have shown that fungicides belonging to the chemical group of benzamides and carbox-amides, succinate dehydrogenase inhibitors (SDHIs), have the potential to inhibit the human SDH en-zyme (Bénit et al. 2019). Some studies have recently raised concerns about the safety of agrochemicals, particularly SDHI fungicides, which are widely used around the world to control fungi in various agricul-tural crops (Van Vranken and Rutter 2015; Bénit et al. 2019; Brenet et al. 2021).
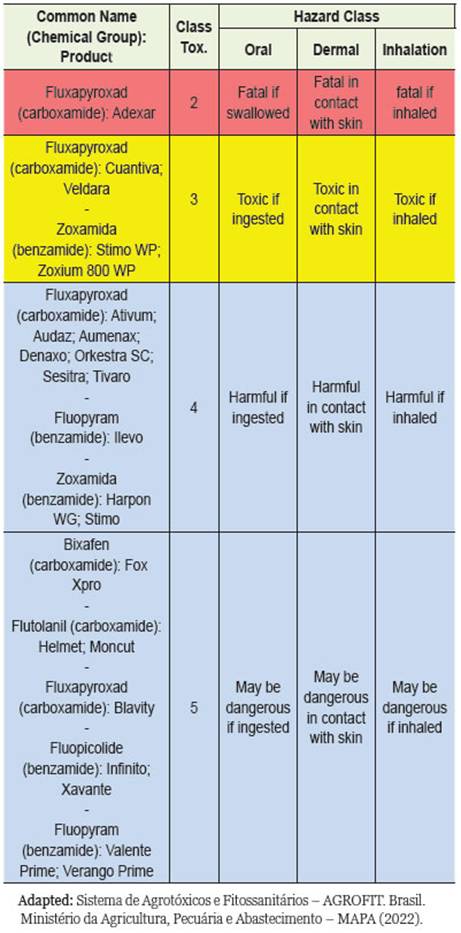
According to the toxicological reclassification of pes-ticides by (Min. da Saúde 2022a), the toxicological and environmental class of the SDHIs found were: Category 2 -Highly Toxic Product - red band; Catego-ry 3 - Moderately Toxic Product - yellow band; Cat-egory 4 -Low Toxic Product - blue belt; and Category 5 -Product Unlikely to Cause Ac ute Injury - blue belt. The degree of toxicity of the substances that make up the SDHIs varies depending on the category (Table 1). Studies report the use of SDHIs in the main agricultural crops: almond, potato, tomato, grape, strawberry, apple, kiwi fruit, cucurbits, oil seed rape, cucumber, corn, barley, tulip bulbs, asparagus, wheat and barley (Sierotzki and Scalliet 2013).
Studies carried out by Collotta et al. (2013); Cao et al. (2019); Dalla Pozza et al. (2019) point out that fungicides can cause DNA mutations and affect gene expression through epigenetic mechanisms. Other studies developed by Van Vranken et al. (2015), Bé-nit et al. (2019), Brenet et al. (2021), because of the universal role of SDH in cellular respiration and mi-tochondrial metabolism in living organisms, exposure to SDHI fungicides can cause adverse health outcomes in humans.
In this review, we integrate recent advances in the medical and agricultural implications of SDH, with the aim of describing the relationship between exposure to SDHI used in Brazil and the epigenetic regulation of SDH associated with the development of gastrointestinal stromal tumor, paraganglioma, and cancer.
MATERIAL AND METHODS
Literature revisión
A search string search was performed using PubMed, with combinations of terms from the following cat-egories: (a) succinate dehydrogenase inhibitors, AND and OR (b) epigenetic regulation of succinate dehy-drogenase. The selection of references was based on studies that evaluated the consequences of SDHI fungicides in human health, and the epigenetic regula-tion of SDH associated with human diseases.
The work consisted of a bibliographic design, and the survey was limited to studies published from 2011 onwards. The searches were limited to the last ten years to allow the analysis of the works most current. The following were established as inclusion and ex-clusion criteria for selection: 1. Selection by the title criterion (relationship of the words that appear in the title with the subject of the review); 2. Selection cri-teria for the selected abstract (the selected study pri-oritizes the objective of the review); 3. Selection by full-text assessment for eligibility (methodology used is adequate, results relevant to the area); 4. Overview of selected articles (impact factor).
Additional references were included because they present relevant information on the classification of SDHIs and adverse results of agrochemicals to human health and the environment, through searches in the Sistema de Agrotóxicos e Fitossanitários (AGROFIT) of the Ministério da Agricultura, Pecuária e Abastec-imento (MAPA), the Agencia Nacional de Vigilancia Sanitária (ANVISA), the Instituto Nacional de Cáncer (INCA) and the Comité Brasileiro de Aqao a Resistencia a Fungicidas (FRAC-BR).
This review summarizes the current state of knowl-edge about the risks of SDHI exposure in non-tar-get organisms and the epigenetic regulation of SDH, drawing conclusions and making recommendations for future research. It is critical to note that review articles, like other types of scientific articles, are a type of research that uses bibliographic or electron-ic sources of information to obtain research results from other authors in order to theoretically support a specific topic (Botelho 2011).
Table 1: Classification of SDHI fungicides found in the review, based on the degree of toxicity of these substances, by the toxicological reclassification of pesticides by ANVISA (2019).
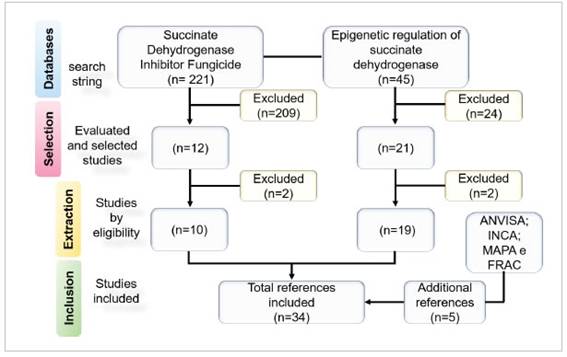
Figure 1: Flow diagram of the selection of studies in the period 2011-2021.
RESULTS
Selection of studies obtained from the research
The initial search resulted in 266 records, and four additional references were selected for detailed evalu-ation (Figure 1). After selection, 241 articles were ex-cluded for the following reasons: 209 for not present-ing any description of SDHIs associated with human disease (n = 12) and 24 for lack of data on epigenetic mechanisms involved in SDH regulation (n=21).
In the extraction stage, 10 selected articles on SDHI fungicides were included in the review (n=10) and two articles were excluded, and of the 21 articles on the epigenetic regulation of SDH, two articles were excluded because they did not specify the epigenetic mechanism involved in the development of human disease (n=19).
Of the 33 articles selected, 29 articles were eligible for inclusion because they included results related to the topic addressed, all written in English. In addition to the references obtained by the search string, publica-tions from ANVISA, INCA, FRAC-BR and MAPA (Min. da Saúde 2022a; and 2022b; FRAC 2022; MAPA 2022) and one publication narrative review (Botelho 2011) were also included in this review. Finally, a total of 34 studies were included in the present analysis.
Classification of SDHIs according to ANVISA, INCA and MAPA In the Sistema de Agrotóxicos e Fitossanitários -AGROFIT of MAPA (Min. da Saúde 2022b) for the chemical group (carboxamide), the following SDHIs were discovered: biixafem, flutolanil, and fluxapy-roxad with formulation in concentrated suspension or emulsifiable concentrate, and fluopicolide, fluopyram, and zoxamide. benzamide chemical group in concen-trated suspension, water-dispersible granules, and wettable powder.
The common name SDHI fungicide Bixafen classified in category 5 as a product unlikely to cause acute dam-age as determined by ANVISA, in the work developed by Bénit et al. (2019) was shown when glutamine is the major carbon source, the presence of SDHIs leads to time-dependent cell death. This process is significant-ly accelerated in fibroblasts derived from patients with neurological or neurodegenerative diseases due to RC impairment (encephalopathy originating from a partial SDH defect) and/or hypersensitivity to oxidative in-sults (Friedreich ataxia, familial Alzheimer’s disease). On the other hand, Kamp et al. (2021) pointed out that fluxapyroxad SDHIs classified in categories 2, 3, 4 and 5 depending on the commercial product, did not result in changes in succinate or lactate levels after in vivo ex-posure in rats.
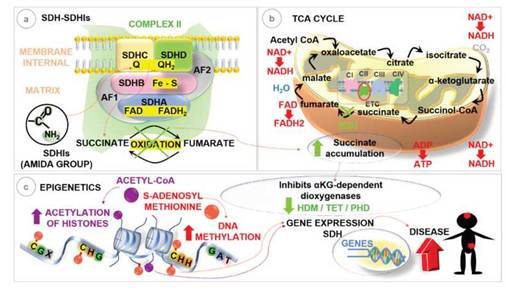
Figure 2: Schematic representation of succinate accumulation and epigenetic changes. (a) SDHIs bind to the SDH enzyme at the binding site of the ubiquinone “Q” subunits SDHB, -C and -D, blocking the oxidation of succinate. (b) Blockade of SDH enzymatic activity by SDHIs will prevent the oxidation of succinate to fumarate in the Tricarboxylic Acid Cycle - TCA, leading to succinate accumulation. (c) Inhibition of aKG-dependent dioxygenases, the histone demethylases (HDM), prolyl hydroxylase (PHD) and the eleven translocation (TET) 5-methylcytosine (5mC) hydroxylases that are directly involved in tumorigenesis, leading to histone alterations and DNA methylation. Adapted: Dalla Pozza et al. (2019); Sierotzki; Scalliet (2013); Xiao etal. (2012); Zhu et al. (2014).
The flutolanil SDHIs classified in ANVISA category 5 (Min. da Saúde 2022a), increased mitochondrial mem-brane potential on exposure to SDHIs in kidney cells. This infers the difficulty of measuring the possible impacts and risks of certain SDHIs on human health, mainly because they are chemical products with differ-ent levels of toxicity, requiring more effective preven-tion and control measures (Van Der Stel et al. 2020).
Exposure to SDHIs and epigenetic mechanisms
In this sense, we represent in schematic how exposure to SDHIs can block the human SDH enzyme (Figure 2a), causing the accumulation of succinate (Figure 2b) and inducing epigenetic changes that regulate gene expres-sion, leading to the development of diseases (Figure 2c). The selected articles demonstrated that the concentra-tion of metabolites produced by the enzymatic reac-tions involved in the TCA cycle, primarily succinate accumulation, controls the epigenetic regulation of SDH by inhibiting several alpha-ketoglutarate (KG)-dependent dioxygenases involved in histone acetyla-tion and methylation and DNA methylation. In this way, we show how SDHIs fungicides can cause epi-genetic changes by inhibiting the human SDH en-zyme (Xiao et al. 2012; Tretter et al. 2016; Sajnani et al. 2017; Bernardo-Castiñeira et al. 2019; Moog et al. 2020; Zhao et al. 2020).
Diseases associated with epigenetic regulation of SDH
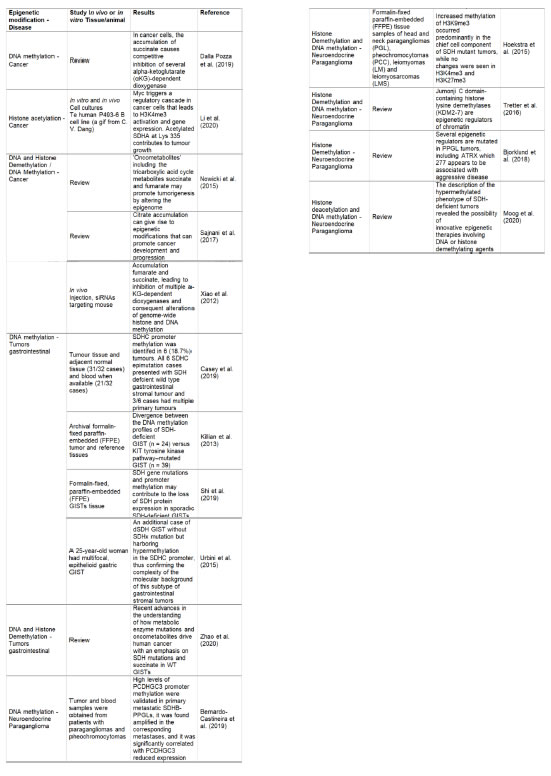
The selected studies revealed that DNA methylation, histone and DNA demethylation are the primary epi-genetic mechanisms that regulate SDH gene expres-sion in cancer, gastrointestinal, paraganglioma, and neuroendocrine tumors. (Table 2).
DISCUSSION
The general structure of SDH
Located on the inner membrane of mitochondria, the SDH holoenzyme is formed by four subunits, SDHA, SDHB, SDHC and SDHD, and two assembly factors, SDHF1 and SDHF2 (Moosavi et al. 2020). The SD-HA subunit catalyzes succinate to fumarate in the TCA Cycle, SDHB is involved in the oxidation of ubi-quinone to ubiquinol in ETC, while SDHC and SDHD are primarily responsible for anchoring the SDH protein in the inner portion of the mitochondrial mem-brane (Zhao et al. 2020).
Table 2: Studies related to epigenetic regulation of SDH associated with cáncer and other diseases.
The TCA cycle generates ATP by glucose oxidation, and metabolites for numerous anabolic pathways. SDH catalyzes one of the eight steps in the TCA cycle (Sierotzki and Scalliet 2013). SDHA oxidizes suc-cinate at the FAD binding site, forming FADH2 and leaving free fumarate to exit the protein. The elec-trons from FADH2 are transferred to the Fe - S clus-ters of SDHB, to the quinone binding site (Qp) in SD-HC and SDHD in the inner membrane, resulting in the total reduction of ubiquinone to ubiquinol (Tretter et al. 2016; Moosavi et al. 2020).
SDHIs in agriculture: Brief history and mode of action
Currently, 22 SDHI compounds are listed in the Brazilian Committee for Action on Fungicide Resis-tance (FRAC-BR): 1. Phenyl-benzamide (Benodanil, Flutolanil and Mepronil); 2. Phenyl-oxo-ethyl thio-phene amide (Isofetamide); 3. Furan-carboxamide (Fenfuram); 4. N-cyclopropyl-N-benzyl-pyrazole-carboxamide (Isoflucipram); 5. N-methoxy-(phenyl-ethyl)-pyrazole-carboxamide (Pidiflumethofen); 6. Oxatin-carboxamide (Carboxin and Oxycarboxin); 7. Pyrazine-carboxamide (Piraziflumide); 8. Pyr-azole-4-carboxamide (Benzovindiflupyr, Bixafen, Fluindapyr, Furametpyr, Impirfluxam, Isopyrazam, Penflufem, Penthiopyrad and Sedaxane); 9. Pyridine-carboxamide (Boscalide); 10. Pyridinyl-ethyl benza-mide (Fluopyram) and; 11. Thiazole-carboxamide (Tifluzamide) (FRAC 2022).
SDHI fungicides have a different structure, but they share an essential common feature, which is the amide bond used for classification. Furthermore, they can be classified into two main categories: (1) those that bind succinate (e.g. malonate) and, (2) those aimed at crop protection, bind ubiquinone (e.g. car-boxamides) (Sierotzki and Scalliet 2013).
The diversity of SDHI fungicides and the biological spectrum exhibited by them is due to the high degree of variation in SDHC and SDHD between species (Si-erotzki and Scalliet 2013). The physicochemical prop-erties of SDHIs allow them to be used in a wide range of applications, including seed treatment, foliar and soil irrigation, for the control of fungi in a variety of crops, e.g. potato, grape, soybean, rice, wheat, corn and others (Tian et al. 2020).
Susceptibility to diseases influenced by the toxic effect of SDHIs The results obtained with the research at AGROFIT for the chemical groups carboxamide and benzamide, which make up the SDHIs fungicides, showed that there are several products registered by MAPA and that they have been used in different MAPA 2022. Fungicides are routinely applied as preventive crop protection, in the various stages of plant growth and in post-harvest storage (Tian et al. 2020).
Among the various fungicides used in agriculture, SDHIs stand out due to their broad spectrum of ac-tion (Sierotzki and Scalliet 2013; Gulkowska et al. 2014). This group of fungicides acts by inhibiting the SDH enzyme of plant pathogens, with SDH being an essential and evolutionarily conserved component of the mitochondrial respiratory chain of living organ-isms (Brenet 2021).
In this context, some concerns have been raised about the agricultural importance of SDH in the de-velopment of fungicides, which may have the poten-tial to inhibit this enzyme not only in plant patho-gens, but also in non-target organisms (Van Vranken et al. 2015; Bénit et al. 2019; Brenet et al. 2022). The SDH enzyme from humans, bees, earthworms, and fungi was sensitive to the eight SDHIs tested, indicat-ing that the SDHB-D subunits that comprise the ubi-quinone reduction site are highly conserved (Bénit et al. 2019).
According to ANVISA, the degree of toxicity of these substances varies from Category 2 - Highly tox-ic product, which can be fatal if ingested, in contact with the skin and/or inhaled, to Category 5 - Product unlikely to cause acute harm (Min. da Saúde 2022a). Is extremely dangerous to compare the toxic effect of SDHIs in vitro with the concentrations of SDHIs ap-plied in agricultural cultivation. According to these authors, the concentration of the fungicide at the spray nozzle outlet and the mist cloud per hectare can be reliably determined. However, the final exposure is determined by a number of uncontrollable factors such as propagation conditions, soil type, vegetation cover, and so on (Bénit et al. 2019).
The neurotoxicity of bixafen, one of the most recently released SDHI fungicides, was assessed, the results found by these researchers through in vivo analy-sis showed that the central nervous system is highly sensitive to bixafen. According to them, this SDHI is neurotoxic in vertebrates and causes defects in neu-rological development, which can cause microceph-aly and impair the growth of motor neuron axons (Brenet 2021). Therefore, strategies to help protect against the neurotoxicity of these substances must be adopted to ensure the protection of human health. Mutations of SDH-encoding genes lead to blockade to varying degrees of SDH activity, being associated with cancer (Zhao et al. 2020) and a wide spectrum of diseases (Hoekstra et al. 2015). However, that pa-tients without mutations in all four SDH subunits may also have diseases caused by the loss of SDH en-zyme activity (Van Vranken et al. 2015). In patients with neurological or neurodegenerative diseases caused by ETC impairment and/or hypersensitivity to oxidative insults, such as Friedreich's ataxia (FR-DA) and familial Alzheimer's disease, a pre-existing mitochondrial defect, such as partial SDH dysfunc-tion, increases susceptibility to SDHIs (FAD) (Bénit et al. 2019).
SDH inhibition may occur as a compensatory mechanism due to the presence of pyruvate, which was sufficient to supply the TCA cycle to the succi-nate oxidation step, limiting NADH depletion. Al-though not statistically significant, flutolanil and mepronil appear to slightly increase mitochondrial membrane potential in kidney cells, while SDH com-pounds increased cellular oxygen consumption, re-sulting in internal mitochondrial hyperpolarization (Van Der Stel et al. 2020).
Results of the characterization analysis of metabolic changes resulting from in vivo exposure to the SDHIs boscalide and fluxapyroxad in rats, indicated that the SDH activity inhibiting action by both compounds did not result in changes in succinate or lactate lev-els. As a result, these authors proposed the existence of multiple biochemical pathways capable of replacing SDH's decreased activity and maintaining homeosta-sis (Kamp et al. 2021).
Inhibiting SDH can limit the ability of the grid to lose carbon in the form of CO2 emissions, forcing it to op-erate with greater carbon efficiency. The simulation of partial SDH inhibition produced similar but more moderate effects than complete SDH inhibition, in-dicating an effect dependence and partial or, in some cases, complete restoration of the flow ranges. Thus, the results found by (Bénit et al. 2019; Van Der Stel et al. 2020; Zhao et al. 2020; Brenet et al. 2021; Kamp et al. 2021) on susceptibility to diseases influenced by the toxic effect of SDHIs, highlight the risks of expo-sure to these fungicides that can contribute to the ac-celeration of disease progression, especially in people who already have a partial deficiency in SDH.
Diseases associated with epigenetic regulation of SDH
Comparing the results presented in the present work (Table 2), we conclude that oncometabolites are involved in the emergence and development of vari-ous tumors and human diseases that involve epi-genetic alterations (DNA and histone methylation demethylation, and histone acetylation). Concerning the negative points, it is observed that more research is required to deepen knowledge in this area, primar-ily aiming at innovative therapeutic strategies for the treatment of cancer and other diseases through clinical trials or in isolated individuals.
Gene expression can be modulated epigenetically through methylation of cytosine residues in DNA and chemical modification of histone tails (Bjorklund and Backman 2018). These processes are essential for normal health, activating and suppressing genes that are vital for cellular functions (Nowicki and Gottliev 2015). Changes in DNA methylation patterns, such as hy-permethylation of CpG islands, have been observed in human cancers, this process being modulated by DNA methyltransferases (DNMTs) that add methyl groups (Nowicki and Gottliev, 2015), and Ten-Eleven translocation (TET) - protein from the dioxygen-ase family that removes methyl groups, converting methyl cytosine (5mC) to 5-hydroxymethylcytosine (5hmC), then 5hmC to 5-formylcytosine (5fC) and finally 5fC to 5-carboxylcytosine (Nowicki and Got-tlieb 2015; Dalla Pozza et al. 2020).
SDH-deficient gastrointestinal stromal tumors (GISTs) exhibit specific clinicopathological features, consist of epithelioid tumor cells, often metastasize to lymph nodes and liver, occur among children and adults, with a female predominance (Shi et al. 2019). The methodology proposed by Casey et al. (2019) for the diagnostic test of SDHC epimutation in GISTs, al-lowed to identify 4 cases of SDHC tumor promoter hypermethylation (Casey et al. 2019).
The case study by Urbini et al. (2015) revealed that epigenetic regulation by DNA methylation of CpG is-lands of the SDHC promoter was observed as an al-ternative mechanism underlying the lack of SDH complex in GIST, he loss or inactivation of SDHB ex-pression in sporadic succinate dehydrogenase defi-cient patients with GISTs is caused by promoter hy-permethylation that can cause gene silencing of the tumor suppressor gene (TSGs) and lead to the forma-tion and development of tumors (Shi et al. 2019).
In the PPGL neuroendocrine tumor containing SDH mutations, a hypermethylator phenotype is associ-ated with downregulation of key genes involved in neuroendocrine differentiation (Aldera and Goven-der 2018). The DNA methylation profile revealed a hypermethylation phenotype in SDHx - PPGLs and revealed that succinate acts as an oncometabolite, in-hibiting 2-oxoglutarate-dependent dioxygenases such as histone and DNA demethylases, causing epigenetic changes in several SDHx - paraganglioma traits (Ber-nardo-Castiñeira et al. 2019).
The epigenetic mechanisms that control cell prolifera-tion and differentiation are regulated by oncometabo-lites, resulting from the enzymatic reactions involved in the energy metabolism of the TCA cycle (Sajnani et al. 2017). Changes in the abundance of metabo-lites such as acetyl-CoA and S-adenosyl methionine (SAM), which are substrates for key biochemical re-actions such as acetylation and methylation, can af-fect the epigenetic status of the entire genome (Ber-nardo-Castiñeira et al. 2019; Zhao et al. 2020). Succinate accumulation inhibits PHDs, resulting in the stabilization of hypoxia-inducible factor-1a (HIFla) proteins. Inhibiting PHD allows HIF sub-units to escape degradation and bind to HIF to form a heterodimer, which forms an active complex under hypoxic conditions and acts on HIF(3 target genes that regulate biological processes such as cell surviv-al, angiogenesis, cell growth, proliferation, and gly-colysis (Sajnani et al. 2017; Moog et al. 2020).
In addition to the currently widely used SDHI fun-gicides, new SDHIs with greater action potential are being tested and developed. In this regard, in terms of health care and the environment, it is recommend-ed to avoid the use of chemical fungicides as much as possible, instead opting for alternative methods of controlling plant diseases, such as biological control and crop rotation, when possible.
CONCLUSIONS
In view of the studies presented in this review, we understand that further in vitro and in vivo to identi-fy the risks of developing cancer, gastrointestinal and neuroendocrine tumors - pheochromocytoma / para-ganglioma associated with SDHI exposure, analyses should be performed to assess susceptibility to dis-eases influenced by the toxic effect of SDHIs.
Based on our interpretation of the articles cited in this review, we discovered that MAPA has recom-mended many SDHI fungicides for various agri-cultural crops, which can significantly benefit pro-duction by controlling plant diseases. Despite their agricultural benefits, SDHIs have been shown in studies to inhibit the SDH enzyme in humans and many other living things.
According to the research presented in this review, the human SDH enzyme is sensitive to some of the SDHIs tested, and the central nervous system is highly sensitive to bixafen, which can cause micro-cephaly and neurodevelopmental defects. Further-more, other research has linked epigenetic regula-tion of the SDH enzyme to the development of cancer, gastrointestinal tumors, and neuroendocrine - pheo-chromocytoma/paraganglioma.
As a result, additional research should be conducted to assess the action potential of SDHIs on the human SDH enzyme, as well as their health and environmen-tal risks. In this regard, the findings of this review high-lighted some recent concerns about the safety of these agrochemicals, which will help with future research to ensure protection against the toxic effects of SDHIs.












 uBio
uBio