Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta Odontológica Latinoamericana
versión On-line ISSN 1852-4834
Acta odontol. latinoam. vol.23 no.3 Buenos Aires dic. 2010
ARTÍCULOS ORIGINALES
Comparative study of physico-chemical properties of MTA-based and portland cements
Álvaro H. Borges1, Fábio L.M. Pedro1, Carlos E.S. Miranda3, Alex Semenoff-Segundo1, Jesus D. Pécora2, Antônio M. Cruz Filho2
1Department of Endodontics, Faculty of Dentistry, University of Cuiabá (UNIC), Cuiabá, MT, Brazil.
2Department of Restorative Dentistry, Faculty of Dentistry of Ribeirão Preto, University of São Paulo (USP), Ribeirão Preto, SP, Brazil.
3Department of Chemistry, University of Ribeirão Preto, Ribeirão Preto (UNAERP), SP, Brazil.
CORRESPONDENCE Alvaro Henrique Borges University of Cuiaba, Faculty of Dentistry Rua Beira Rio 3100, Jd. Europa, 78.065-480 Cuiaba, MT, Brazil Telephone +55-65-3363-1000, Fax: +55-65-3623-3777 e-mail: ahborges@brturbo.com.br
ABSTRACT
The purpose of this investigation was to evaluate the physicochemical properties of gray and white structural and nonstructural Portland cement, gray and white ProRoot MTA and MTA BIO. The water/powder ratio, setting time, solubility and pH (hydrogen-ion potential) changes of the materials were evaluated. Tests followed specification #57 from the American National Standard Institute/ American Dental Association (2000) for endodontic sealing materials and pH was determined by a digital pHmeter. The test results were statistically analyzed by variance analyses for global comparison and by the complementary Tukey’s test for pairwise comparisons (5%). Considering the water/powder ratio, no significant difference (p>0.05) was observed among the cements. MTA BIO (33.10±2.30) had the lowest setting time (p<0.05), gray Pro- Root MTA (10.10±2.70) had the highest (p<0.05). White nonstructural Portland cement (2.55±0.08) had the highest solubility (p<0.05), while gray ProRoot MTA (1.03±0.12) had the lowest (p<0.05), although all materials showed solubility values in compliance to ANSI/ADA. No difference (p>0.05) was observed among materials when considering pH evaluation. The pH levels were highly alkaline immediately after immersion in solution, remaining stable throughout the test period. The authors conclude that the cements had similar water/powder proportions. MTA BIO had the shortest setting time and gray ProRoot MTA had the lowest solubility. All cements had similar behavior in the pH analysis.
Key words: Endodontics; Mineral Trioxide Aggregate; Root Canal Filling Materials.
RESUMO
Estudo comparativo das propriedades físico-químicas dos cimentos portland e a base de MTA
A proposta desse trabalho foi de avaliar as propriedades fisicoquimicas dos cimentos Portland cinza e branco estrutural e nao estrutural, do ProRoot MTA cinza e branco e do MTA BIO. Foram avaliadas a proporcao po-liquido, tempo de endurecimento, solubilidade e variacao do pH dos cimentos. Os testes seguiram as normas que determinam a especificacao numero 57 da ANSI/ ADA para cimentos obturadores e a variacao de pH foi analisada por meio de pHmetro digital. Os resultados dos testes foram analisados estatisticamente por meio de teste de analise de variancia e pelo teste de Tukey para comparacao entre pares, com nivel de significancia de 5%. Nao foram evidenciaram diferencas estatisticamente significantes (p>0,05) entre os cimentos no que se refere a proporcao po-liquido. O MTA BIO (33,10±2,30) mostrou os menores valores de tempo de endurecimento (p<0,05), enquanto o ProRoot MTA cinza (102,10±2,70) mostrou os maiores resultados (p<0,05). Todos os materiais mostraram valores de solubilidade dentro dos padroes da ANSI/ADA, sendo que o cimento ProRoot MTA cinza (1,03±0,12) apresentou a menor solubilidade (p<0,05) e o Portland branco nao estrutural (2,55±0,08), a maior (p<0,05). No que se refere a variacao do pH, nao foram determinadas diferencas significantes entre os materiais (p>0,05). Os niveis de pH mostraram-se altamente alcalinos, imediatamente apos a imersao em agua, mantendo-se estavel ao longo do periodo de teste. Concluiu-se, que os cimentos estudados tiveram proporcoes po-liquido similares. O MTA BIO apresentou tempo de endurecimento mais curto e a menor solubilidade foi apresentada pelo ProRoot MTA cinza. Todos os cimentos tiveram comportamento semelhante na analise do pH.
Palavras chave: Endodontia; Mineral Trioxido Agregado; Materiais Restauradores do Canal Radicular.
INTRODUCTION
Mineral Aggregate Trioxide (MTA) was developed as a root-end filling material in the 1990s and later accepted by the US Federal Drug Administration, becoming commercially available as ProRoot MTA (Tulsa Dental Products, Tulsa, OK, USA)1. Studies showed that it has excellent physical2,3, chemical4-6 and biological properties7,8. The manufacturer initially emphasized that the material was made up of 50-75%, by weight, calcium oxide and 15-25% silicate dioxide. It later became clear that it was very fine, ordinary Portland cement, type 1, containing bismuth oxide as a radiopacifying agent4,9,10.
MTA and Portland cements have similar antimicrobial action9, no difference in biocompatibility11,12 and the same tissue response when used in pulpotomies13. Considering this similarity and its low cost, Portland cement is a great possibility for substituting MTA cement. Although their biological properties are similar, great differences between Portland cement and MTA have been reported, especially considering physico-chemical characteristics and the characteristics of the material mass14. With the aim of improving the desirable properties of these cements, derivatives are offered in the market. MTA Angelus (Angelus Solucoes Odontologicas, Londrina, Brazil), for example, is composed of 80% Porltand cement and 20% bismuth oxide5,15. MTA has recently become available in two formulations: white and gray4,6. The white cement has a similar composition to the gray, expect for the presence of iron oxide16. Similarly, Portland cement is available as white and gray, with the white classified as structural and nonstructural, according to the carbonate material in its composition17.
Portland cement is presented as a possible alternative to MTA indications. However, before being used clinically, studies should be conducted to elucidate its physical, chemical and biological properties, and its effects on the human body. It is thus mandatory to conduct studies to elucidate the physico-chemical properties of these cements. The aim of the present study was to determine the water-powder ratio, setting time, solubility and hydrogen ion potential of gray and white structural and non-structural Portland cement, ProRoot MTA and MTA BIO.
MATERIALS AND METHODS
The materials used in this study are described in Table 1. The water/powder ratio was initially established by weighing 3 g of cement and mixing it with 0.20 mL of distilled water. The quantity of powder not used was weighed from the initial quantity. This procedure was repeated five times for each material. The setting time and solubility of the cements were determined according to methods prescribed by specification #57 of the American National Standard Institute/ American Dental Association18 for endodontic sealing materials and as suggested by Carvalho Jr. et al. (2007)19, allowing the reduction of 80% in volume of material for conducting tests, without involvement or interference in results. For setting time, 5 stainless steel molds, with 10 mm-inner diameter and 2 mm-uniform thickness were made for each material. The cement was mixed and inserted into the metallic molds. After 120 ± 10 seconds from the beginning of the mixture, the set formed by the glass plaque/metallic mold/cement was left in a plastic recipient with hermetic sealing and maintained at a constant temperature of 37 ± 2°C and 95 ± 5% air humidity, inside an incubator (Olidef, Ind. e Com. Aparelhos Hospitalares, Ribeirao Preto, SP, Brazil), until the end of the test. After 150 ± 10 seconds from the beginning of the mixture, a Gillmore needle (100 ± 0.5 g and 2 ± 0.1 mm active tip) was vertically lowered into the horizontal surface of the material. The needle was inserted at regular intervals of 60 seconds until the indentations could not be observed on the cement surface. The setting time was determined as the time from the beginning of the mix until the time at which the indentations were not visible on the cement surface.
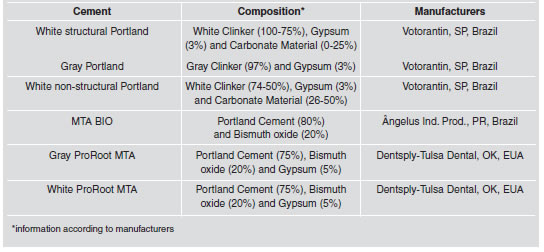
Table 1: Tested materials and compositions.
For the solubility test, a total 5 samples (1.5 mmthickness and 7.75 mm-inner diameter) were used for each material. The cement was prepared and inserted into the mold. In sequence, a 0.5 mm-diameter waterproof nylon was inserted in the softened cement. After three times the setting time, the sample was removed from the mold and weighed on a precision scale of 0.0001 g (Ohaus Corporation, New Jersey, NJ, USA). The sample suspended by the nylon was placed in a wide-mouthed plastic recipient containing 7.5 mL of distilled water, avoiding the contact with the internal wall. This container was maintained hermetically closed and placed in an incubator at a constant temperature of 37 ± 2°C for 24 hours. After this time, the sample was removed and the excess water removed with absorbent paper. The sample was maintained in dehumidifier for 24 hours, after which it was weighed a second time. The material’s solubility was considered as the percentage of lost mass compared to the initial mass. Five repetitions were considered for each material. Samples were obtained in the same way to evaluate hydrogen ion potential. A total 5 samples (1,5 mm thickness and 7,75 mm inner diameter) were used for each material. Each cylinder was sealed in a flask containing 7.5 mL of distilled water. Distilled water pH measurements (PH30 Sensor Corning; Corning Inc, New York, NY, USA) were taken with a pH meter at 1/4, 1/2, 1, 2, 3, 4, 6, 9, 12, 24, 48, 72, 96, 144 and 168 hours after spatulation. During the experiment, pH was analyzed for each sample in the same plastic recipient without liquid substitution. It was measured five times for each material.
Mean values and standard deviations were recorded for all measurements. Statistical analyses were carried out for setting time, solubility and pH change using ANOVA and Tukey’s test at 5% level of significance. When sample distribution was nonnormal, nonparametric analysis of variance were performed with Kruskal-Wallis test (α=0.05).
RESULTS
Results showed no statistical difference (p>0.05) in the water/powder ratio, among the cements analyzed. Table 2 shows the amount of powder needed, in grams, when mixed into 1 mL of distilled water. Setting time (Table 3), in minutes, showed statistically significant differences among the cements (p<0.05). MTA BIO had the lowest values, while gray ProRoot MTA had the highest. No difference was observed (p>0.05) between white structural and non-structural Portland cements, although they were different (p<0.05) from the others.
Table 2: Water/powder ratio of the materials.
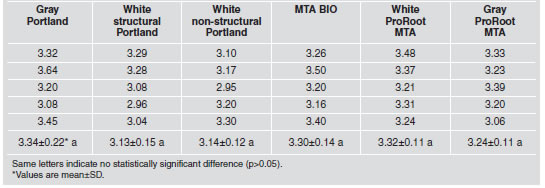
Table 3: Setting time (in minutes) of the tested materials.
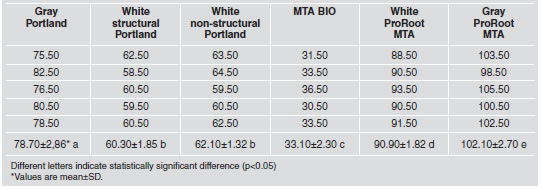
White non-structural Portland cement had the highest mean value for solubility (Table 4), while gray ProRoot MTA the lowest, showing a statically significant difference (p<0.05) from the others materials. Gray Portland, white structural Portland cement, MTA BIO and white ProRoot MTA were similar (p>0.05), with intermediate values.
Table 4: Solubility (percentage) of the tested materials.
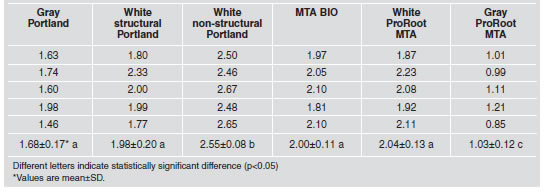
Table 5 summarizes the mean pH values recorded for the materials. The pH values for the cements ranged from 10.92 to 11.77 (Fig. 1), with no significant differences (p>0.05).
Table 5: pH values recorded according to material and period of time.
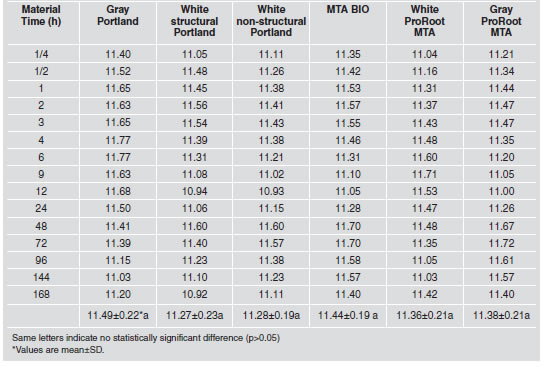
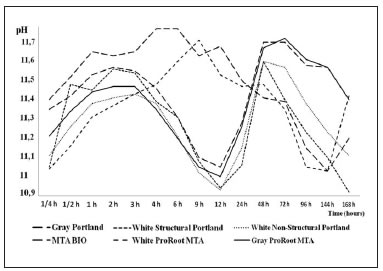
Fig. 1: Changes in the pH of cements over time.
DISCUSSION
ProRoot MTA and MTA BIO patents describe these materials as Portland cement, with gypsum addition for setting time control14 and bismuth oxide for radiopacity improvement9,10. Portland cements are manufactured by clinker incineration, produced by calcination or raw material burning with calcareous, iron, silica and steel, at high temperatures1. The proportions of the constituents are carefully controlled for the production of five main types of Portland cement and numerous subtypes17. White Portland cements are manufactured by removing tetra-calcium aluminoferrite from gray cements1,16 and classified as structural or non-structural, according to the amount of carbonate material in their constitution17.
MTA-based and Portland cements are hydrophilic and harden in presence of water20; however, the strength of Portland cements decreases with decreasing water/powder ratio21. For Mta-based cements, manufacturers recommend a water/powder ratio of 3:11,3. However, this proportion results in a very fluid mix21, hindering application. In this case, some authors22 recommend that the material be placed under gauze until it acquires consistency suitable for use. Given the lack of a standardized ratio, a mean water/powder ratio of 3.13 to 3.33 g of powder to 1 mL of water was established, showing that the amount of powder in the same bulk of water was close for different materials. Thus, mean values were determined, i.e., 3.24 g of powder to 1 mL of water, standardizing the tests9,10. Portland cement is composed of gypsum (3%) and clinker (97%), which in turn uses limestone and clay as raw materials17,20. Gypsum is added by cement manufacturers to retard cement clinker setting time1. In this study, white structural and non-structural Portland cements presented similar setting times, which were lower than the setting time for gray Portland cement. This is related to fact that gray Portland cement is made up of 100% clinker and gypsum, while white Portland cement contains 25 to 50% of carbonate material, decreasing the percentage of gypsum in the bulk17.
MTA BIO had the lowest setting time, while gray ProRoot MTA had the highest. Although both are MTA based cements, MTA BIO consists of 80% Portland cement and 20% bismuth oxide5,15. In addition to these two components, ProRoot MTA includes 5% gypsum in its composition23, retarding setting time. In relation to white and gray ProRoot MTA, it is interesting to highlight that white ProRoot MTA hardened more quickly than gray, as was also observed in Portland cements. It is suggested that the raw material base that consists of white ProRoot MTA probably contains white Portland6,24, and the same relationship is expected with regard to gray cements. Due to the long setting time of ProRoot MTA, the addition of hydroxides and aluminates has been recommended to accelerate cement setting25. It was observed in this study that gray ProRoot MTA was less soluble than others, which is consistent with others authors5,26. The striking difference, considering solubility, between gray ProRoot MTA and other cements may be related to chemical composition, which showed different structures after the hardening reaction20. Lower setting time may be one of the reasons for the greater solubility of Portland cement27. Gypsum, which retards the hardening of the cement, allows a long time for the accommodation of the particles that make up cement, leading to the formation of a durable and more resistant structure20. Moreover, bismuth oxide, which is water insoluble, is also added to MTA, contributing to the reduction in solubility21. White non-structural Portland cement solubility was significantly greater than others. The solubility of this cement is related to a lower content of clinker in its constitution17. The resulting structure would therefore be less resistant, which would explain greater release of calcium ions, the main species in the composition of the material15. White structural Portland cement, white ProRoot MTA and MTA BIO have similar solubility, which is related to the materials in their compositions, which are very similar, i.e., MTA cements consist of 75 to 80% of Portland cement. Thus, the chemicals that could influence and change solubility are common to all materials5,10,16. It is important to emphasize that cement solubility value complied with specification #57 of the American National Standard Institute/ American Dental Association19 (2000) for endodontic sealing materials requirement, as it did not exceeded 3% mass fraction. Analyses of hydrogen ion potential showed that pH values were alkaline for all cements, 15 min after water immersion, remaining high until the last reading (168 h). The setting reaction of cement is based on the reaction of cement anhydrous compounds with water, in which individual components of cement are attacked and react to form hydrated compounds20. Hydration is primarily a hydrolysis of silicates, producing a hydrate of lower basicity calcium silicate (CHS gel); releasing lime is separated in the form of calcium hydroxide13: calcium silicates forming CHS gel and calcium hydroxide21. Thus, these cements can be considered, after hardening, as calcium hydroxide contained in a silicate matrix. Calcium hydroxide is responsible for the high alkalinity of cement1,28. Immediate increase in pH after material immersion is due to the reaction that takes place when cement comes into contact with water, resulting in a saturated calcium hydroxide solution6,15,28. Preliminary studies15 reported lower values than those observed in this work, and the difference is probably due to methodology used, since the researchers changed the water for each measurement performed. Thus, after each reading, the pH of the solution returned to a value close to that of distilled water, requiring a longer time to reach a higher pH.
Based on these results, is questionable to suggest substituting MTA-based cements with Portland cements in endodontic treatments, as recommended in many studies11,29. It is important to know how the materials behave regarding biocompatibility and microbial activity, and also in vivo experiments for adequate indication and security, when used in humans.
1. Camilleri J, Pitt Ford TR. Mineral trioxide aggregate: a review of the constituents and biological properties of the material. Int Endod J 2006;39:747-754. [ Links ]
2. Wilkinson KL, Beeson TJ, Kirkpatrick TC. Fracture resistance of simulated immature teeth filled with resilon, gutta-percha or composite. J Endod 2007;33:480-483. [ Links ]
3. Nandini S, Ballal S, Kandaswamy D. Influence of glassionomer cement on the interface and setting reaction of mineral trioxide aggregate when used as a furcal repair material using laser raman spectroscopic analysis. J Endod 2007;33:167-172. [ Links ]
4. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J 2005; 38:834-842. [ Links ]
5. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray Pro Root MTA, white ProRoot MTA, and gray MTAAngelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;102:806-815. [ Links ]
6. Islam I, Kheng Chng H, Jin Yap AU. Comparison of the physical and mechanical properties of MTA and Portland cement. J Endod 2006;32:193-197. [ Links ]
7. Chong BS, Pitt Ford TR, Hudson MB. A prospective clinical study of mineral trioxide aggregate and IRM when used as root-end filling materials in endodontics surgery. Int Endod J 2003;36:520-526. [ Links ]
8. Shahi S, Rahimi S, Lotfi M, Yavari HR, Gaderian AR. A comparative study of the biocompatibility of three root-end filling materials in rat connective tissue. J Endod 2006; 32:776-780. [ Links ]
9. Estrela C, Bammann LL, Estrela CRA, Silva RS, Pecora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J 2000;11:3-9. [ Links ]
10. Funteas UR, Wallace JA, Fochtman EW. A comparative analysis of mineral trioxide aggregate and Portland cement. Aust Endod J 2003;29:43-44. [ Links ]
11. Saidon J, He J, Zhu Q, Safavi K, Spangberg LSW. Cell and tissue reactions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:483-489. [ Links ]
12. Bernabe PFE, Gomes-Filho JE, Rocha WC, Nery MJ, Otoboni- Filho JA, Dezan Jr E. Histological evaluation of MTA as a root-end filling material. Int Endod J 2007;10:1-8. [ Links ]
13. Holland R, Souza V, Murata SS, Nery MJ, Bernabe PFE, Otoboni Filho JA, Dezan Jr E. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J 2001; 12:109-113. [ Links ]
14. Dammaschke T, Gerth HUV, Zuchner H, Schafer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mat 2005;21:731-738. [ Links ]
15. Duarte MAH, Demarchi ACCO, Yamashita JC, Kuga MC, Fraga SC. pH and calcium ion release of 2 root filling materials. Oral Sug Oral Med Oral Path Oral Radiol Endod 2003;95:345-347. [ Links ]
16. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod 2005;31:101-103. [ Links ]
17. Brazilian Association of Portland Cement. Technical Guide number 106. Guia basico de utilizacao do cimento Portland. Sao Paulo. 2002 [ Links ]
18. American National Standard Institute/ American Dental Association. Specification n. 57. USA.2000 [ Links ]
19. Carvalho Jr JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa Neto MD. Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. J Endod 2007;33:1110-1116. [ Links ]
20. Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J 2007;40:462-470. [ Links ]
21. Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water/powder ratios. J Endod 2003;29:814-817. [ Links ]
22. Nekoofar MH, Haddad DC, Nolde J, Aseeley Z. Water content of ampoule packaged with ProRoot MTA. Int Endod J 2009;42:549-533. [ Links ]
23. Ferris DM, Baumgartner JG. Perforation repair comparing two types of mineral trioxide aggregate. J Endod 2004; 30:422-424. [ Links ]
24. Islam I, Chng HK, Yap AUJ. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J 2006;39:220-225. [ Links ]
25. Bortoluzzi EA, Souza EM, Reis JMSN, Esberard RM, Tanomaru-Filho M. Fracture strength of bovine incisors after intra-radicular treatment with MTA in an experimental immature tooth model. Int Endod J 2007;40:684-691. [ Links ]
26. Chng HK, Islam I, Yap AUJ, Tong YW, Kob ET. Properties of a new root-end filling material. J Endod 2005;31:665-668. [ Links ]
27. Danesh G, Dammaschke T, Gerth HUV, Zandbiglari T, Schafer E. A comparative study of selected properties of ProRoot mineral trioxide aggregate and two Portland cements. Int Endod J 2006;39:213-219. [ Links ]
28. Camilleri J. The physical properties of accelerated Portland cement for endodontic use. Int Endod J 2008;41:151-157. [ Links ]
29. Duarte MAH, Demarchi ACCO, Yamashita JC, Kuga MC, Fraga SC. Arsenic release provided by MTA and cement. Oral Sug Oral Med Oral Pathol Oral Radiol Endod 2005; 99:648-650. [ Links ]














