INTRODUCTION
Fixed orthodontic appliances are one of the most common ways to treat dental malocclusions. Given their retentive nature, these appliances have been associated to increased dental biofilm in the oral cavity 1-4 . Dental biofilm is made up of múltiple species of acidogenic bacteria related to dental caries progression such as Lactobacillus spp. and Streptococcus mutans (S. mutans). These bacteria produce acids as a byproduct of fermentable carbohydrate metabolism, which in a favorable oral environment can lead to demineralization of the tooth surface 5 . The adherence and colonization of these microorganisms during orthodontic treatment is influenced by various factors such as bracket material, its physical properties, the presence and type of ligatures, and the patient’s oral hygiene habits 3 . Microbial interactions between the surface of the brackets and the oral microbiota are due in part to the amount of surface free energy (SFE) in the bracket material, which is a determining factor in the degree of bacterial adhesion and therefore also in the accumulation of dental biofilm 6 . Dental literature suggests a positive correlation between adherence of S. mutans and orthodontic materials with high SFE 7 . However, the findings are contradictory: Eliades et al. reported higher SFE in metal brackets compared to ceramic brackets, but Lee et al. found the opposite 7, 8 .
Normally, the oral microbiome is in balance, but the presence of fixed orthodontic appliances can cause a pathological imbalance that leads to a demineralizing process in tooth enamel 5 . A meta-analysis by
Sundararaj et al. found that the incidence of white spot lesions in patients with orthodontic treatment was 45.8%, which suggests that fixed orthodontic appliances are associated with increased presence of S. mutans and an uncontrolled decrease in salivary and oral pH, which are necessary etiological factors for the initial development of carious lesions 5, 9 . Some of the most important factors to evaluate when planning an orthodontic treatment include individual patient risk assessment based on their oral hygiene habits, microbiological and salivary conditions, dental caries experience and the presence of periodontal disease, among others. According to Bonetti et al. (2013), salivary properties are not affected by the presence of fixed orthodontic appliances 10 ; nevertheless, Arab et al. (2016) concluded that there are salivary and microbiological changes associated with the presence of fixed orthodontic appliances 11 .
In an in vitro study, Brusca et al. observed that S. mutans adherence was greater on non-metal brackets (composite and ceramic) than on metal brackets 12 . However, in a similar study, Pappaionnau et al. did not find any difference in bacterial adherence related to bracket material, and therefore recommended conducting an in vivo study to determine whether there is a clinically important difference among various types of brackets, and their effect on the presence of S. mutans 13 .
Although some studies have been published on the subject, there is still no conclusive evidence about the relationship between the number of salivary S. mutans colony-forming units (CFU) in patients undergoing fixed orthodontic treatment and the different types of bracket material, compared to patients without orthodontic appliances. Thus, the aim of this study was to determine the salivary S. mutans CFU/mL count in participants with and without fixed orthodontic appliances.
MATERIALS AND METHOD
This was a cross-sectional study on 21 participants. Previous publications were used as a reference for sample size 10, 11 . Applying a non-probability convenience sampling method, individuals were invited to participate voluntarily, and those selected for the study were all of legal age, non-smokers, without orthodontic appliances or with metal and non-metal fixed orthodontic appliances. Subjects who had been under antibiotic treatment within the three months prior to sampling or used any type of dental prosthesis or removable oral appliances were excluded.
The following variables were analyzed: sociodemographic (age, sex, socioeconomic status), orthodontic treatment (absence or presence of appliances, metal or non-metal fixed appliances), oral hygiene (frequency of tooth brushing and use of dental floss and mouthwash), microbiological (S. mutans CFU/mL count), and salivary pH.
Saliva collection
All samples were collected during the morning to avoid possible alterations due to diurnal variations in salivary pH 14 . The participants were instructed to brush their teeth two hours or more before sample collection and not to eat anything after that. Each participant was given a piece of bite rim wax (1 cm 3 ) to chew for three minutes, and two sterile test tubes and funnels. Sufficient time was allotted for the collection of 5 mL of saliva per tube, one for microbiological culture and the other for salivary pH analysis.
Salivary pH analysis
Salivary pH was measured no more than 30 minutes after sample collection using a benchtop pH-meter (Hanna® Instruments HI-2210) that was properly calibrated between each sample. The tip of the electrode was disinfected with fifth-generation quaternary ammonium solution (Benzaldina®). The pH-meter electrode was immersed in the test tube carefully, without touching the bottom, leaving it suspended until the digital measurement was recorded.
Microbiological analysis
The saliva samples were processed in a sterile environment inside a previously decontaminated laminar flow cabinet. Using a calibrated micropipette,
0.5 mL of each sample were aseptically transferred to test tubes with sterile saline solution (4.5 mL), sequentially, to obtain serial dilutions from 10-1 to 10-3(dilution factors were determined based on results collected in a pilot study). After every transfer, each suspension was vortexed for sixty seconds.
The samples were cultured in Petri dishes with Mitis Salivarius Agar (Becton, Dickinson and Company, Difco™) supplemented with 0.2 U/mL of bacitracin and potassium tellurite for selective isolation of S. mutans 15 . The culture media were checked to ensure absence of condensation. A micropipette was used to transfer 100 pl of each dilution, performing duplicate plating and changing the tip between each dilution. Each drop was spread evenly over the agar surface using a sterile Drigalski spatula, sequentially, from the highest to the lowest dilution factor. Seven plates were cultured for each saliva sample: one direct plating and three dilutions in duplicate.
The petri dishes were incubated in anaerobic jars (ThermoScientific™) with anaerobic gas generating sachets (ThermoScientific™ Oxoid AnaeroGen) for 48 hours at 37 °C. S. mutans CFU/mL were counted using a digital colony counter, identifying each colony based on macroscopic characteristics such as color and morphology. In cases of doubt, Gram staining was used.
The information obtained was entered in duplicate in two excel databases and validated using the Epidata 3.1 software. Any discrepancies were corrected, and a fully refined database was exported to the Stata I/C version 14.0 statistical package for analysis.
Statistical analysis
The univariate analysis of the qualitative variables consisted of calculating absolute frequencies and percentages. For quantitative variables, measures of central tendency and dispersion were calculated. In the bivariate analysis, Fisher’s exact test was applied for qualitative variables, and Kruskal Wallis test was used for quantitative variables, as required, considering that the salivary pH and CFU count variables did not have normal distribution. Dunn’s test was used as a post-hoc test for multiple comparisons. Statistical significance was considered for p-values lower than 0.05 (p<0.05).
Ethical considerations
This study was approved with the Ethical Concept 05222014 by the Ethics in Research Committee of the School of Dentistry at Universidad Santo Tomás (Colombia). It was classified as “research with minimum risk” according to Resolution 8430 of October 1993, which establishes the scientific, technical and administrative standards for health research in Colombia 16 . All participants signed an informed consent form after receiving an explanation of the aim and procedure of the study. The principles of respect for autonomy, beneficence, justice and nonmaleficence were applied.
RESULTS
Of the 21 participants, 14 (66.7%) were females, and all were university students. Mean age was 20.4 ± 2.2 years [Me: 20, IQR: 19 - 21] and ranged from 18 to 27 years. No statistically significant difference in age was found when comparing the absence and presence of orthodontic appliances (metal and non-metal) (p=0.9819).
There was no statistically significant difference between groups in relation to sex and socioeconomic status, as shown in Table 1. Middle socioeconomic status was the most frequent in all groups (p=0.594). Regarding oral hygiene, the median for tooth brushing was three times a day for all three groups, the median for use of dental floss was once a day, which corresponded to the group with metal appliances, and the median for use of mouthwash was once a day for all groups ( Table 2 ).
Table 1 Frequency distribution of sex and socioeconomic status in the appliance-free, metal appliance, and non-metal appliance groups
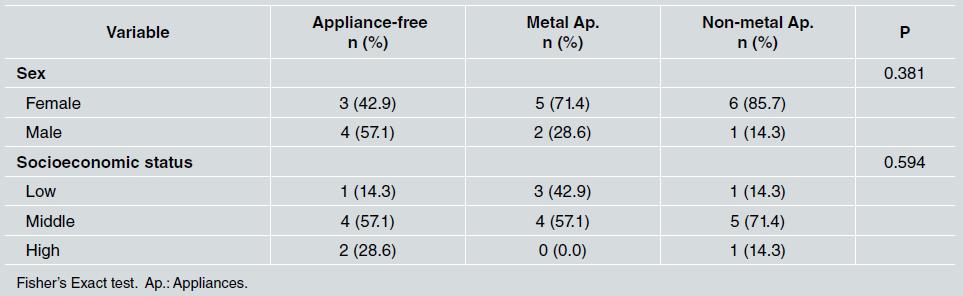
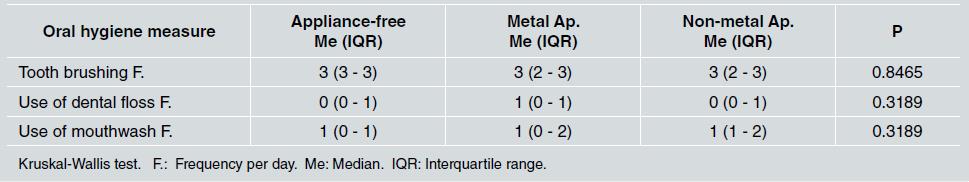
Table 2 Median and interquartile range of daily oral hygiene measures reported in the different groups
In relation to salivary pH, the median was lower [Me: 7.3, IQR: 7.0 - 7.4] in the group with metal appliances than in the other groups, with a statistically significant difference (p=0.0478). Furthermore, statistically significant differences were found between not having any orthodontic appliance and having metal appliances (p=0.0239), and between having metal and non-metal appliances (p=0.0118) ( Table 3 ).
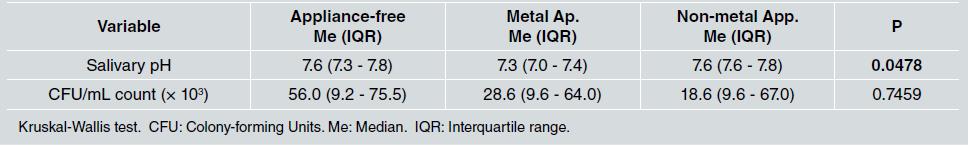
Table 3 Median and interquartile range of salivary pH and salivary S. mutans CFU/mL count in the appliance-free, metal appliance, and non-metal appliance groups
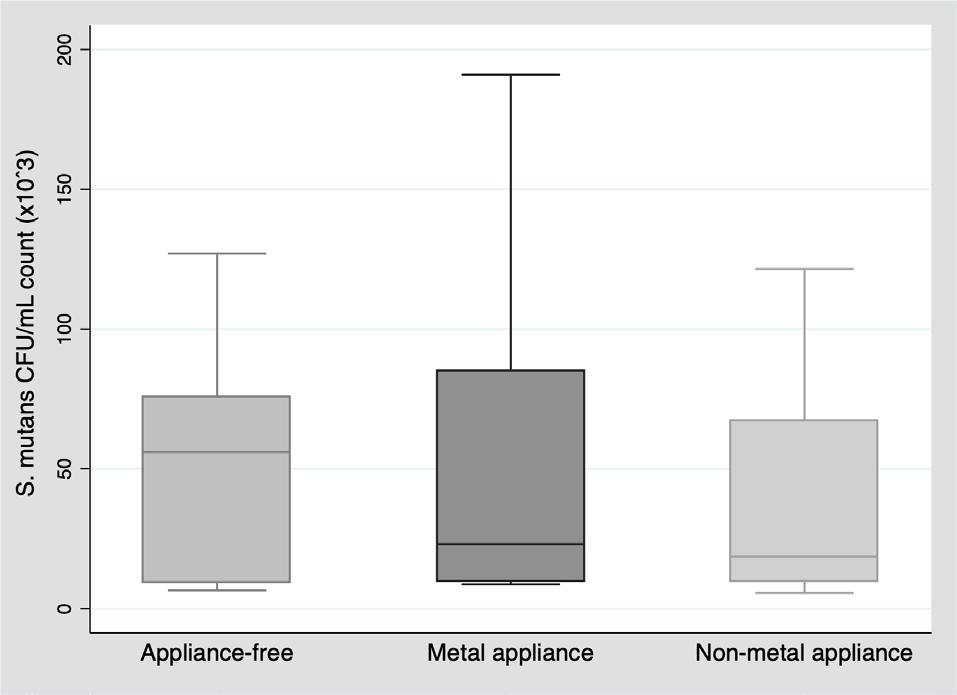
For S. mutans CFU/mL count, no statistically significant difference was observed (p=0.7459), although the median count was lower in the two groups with orthodontic appliances than in the appliance-free group (Table 3, Fig. 1).
DISCUSSION
The S. mutans CFU/mL count did not differ significantly between groups (appliance-free, metal appliances and non-metal appliances). Nonetheless, a statistically significant difference was found in saliva pH levels between the group without appliances and the group with metal appliances, as well as between the groups with metal and non-metal appliances, possibly due to lower saliva pH levels in the group with metal appliances. Papaioannou et al. 13 conducted an in vitro investigation to evaluate the adhesion of S. mutans to the surfaces of three types of brackets (six metal, six ceramic, and six plastic) covered with a salivary pellicle. Although a higher number of S. mutans CFU was found on metal brackets, the difference was not statistically significant (p=0.360). The authors suggest that the affinity of S. mutans to one material or another cannot be confirmed because it is the salivary properties that influence this adhesion. They also mention that Streptococcus sanguis may have an antagonistic relationship with S. mutans , possibly preventing its adhesion 13 . In the present study, the salivary S. mutans CFU/mL count was higher in the group with metal appliances [Me: 28.6*10 3 ] than in the group with non-metal appliances [Me: 18.6*10 3 ] but the difference was not statistically significant (p=0.8480).
Similarly, in another study, Jurela et al. found no statistically significant difference between salivary S. mutans CFU/mL counts in participants with fixed metal and non-metal appliances, even though the number of colonies was higher in participants with metal appliances 17 , as was the case in the present investigation.
Another factor that affects salivary pH and biofilm organization is the host’s diet 5 ; though it has been challenging to understand the complexity of microbial communities interacting with the nutrients in the diet 18 . The authors of a recent systematic review concluded that fixed orthodontic appliances cause major changes in the oral microbiota one month after being installed, regardless of the difficulty of standardizing the results given the different methods for sample collection and microbial count. None of the 51 prospective studies assessed the patients’ diet, and not all of them presented high methodological quality 4 .
A review by Freitas et al. (2014) found moderate evidence supporting changes in the quantity and quality of the oral microbiota due to the presence of fixed orthodontic appliances, possibly because it included eight articles with samples collected from oral mucosa as well as intraoral appliances. The review also highlights the lack of diet assessment and the impact of individual oral hygiene practices 19 . In our study, all three groups reported good oral hygiene measures, including tooth brushing, use of dental floss and mouthwash, perhaps because all the participants were university students, of whom 13 (61.9%) were in the undergraduate dentistry degree program. These 13 participants were evenly distributed across the three groups.
Regarding salivary pH levels, previous studies recommend caution when reviewing study results because salivary properties may vary depending on age, circadian rhythm, time of day and level of hydration, among other factors 20 . In the current study, salivary pH was measured using a digital pH meter, and all samples were collected during the morning, allowing no more than 30 minutes between collection and measurement. Lower pH values were found in the group with metal orthodontic appliances (p=0.0478), as was also reported by Kanaya et al., who found lower salivary pH levels in participants with orthodontic appliances than in those without orthodontic appliances (p=0.001) 20 .
The morning was chosen as the best time to collect the saliva because many studies reported having conducted this procedure between 7:30 and 11:30 a.m. 21,22, 23 . These studies suggest that collecting saliva samples in the morning helps control fluctuations in salivary microbial counts that occur throughout the day. Petti et al. (2001) also recommend that saliva samples should be collected before eating and tooth brushing 24 , as it was done in the current study.
A limitation of the current study was the sample size. There were 21 participants, in accordance with sample sizes reported in previous studies with similar aims, albeit with different designs. The cross-sectional design used is also a limitation because saliva samples were collected at one specific point in time instead of longitudinally. Another limitation was not having carried out a clinical examination, even though the study did not aim to correlate oral health status with the S. mutans CFU/mL count. Despite these limitations, it is important to note that this study estimated microbial populations using saliva samples to count S. mutans CFU/mL, which involves more methodical and rigorous labor than do microbial counts using bacteria test kits. Moreover, there are advantages to using saliva samples: saliva is easy to collect, the procedure is non-invasive, and there is no requirement to collect saliva from specific sites within the oral cavity, unlike bacterial plaque samples, which are generally site-specific 25 . Nonetheless, excellent agreement has been found between results from stimulated saliva samples and plaque samples for quantitative evaluation of S. mutans 26 .
This study serves as a reference point for further studies on salivary S. mutans CFU count in relation to the presence and absence of metal and non-metal orthodontic appliances, considering that there are currently no publications on the subject in Colombia.