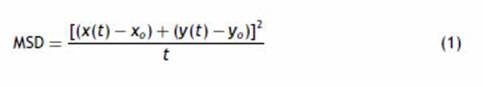Introduction
Proteus mirabilis (P. mirabilis) causes urinary tract infec-tions (UTI), although it does not often colonize the normal unobstructed urinary tract1. It is more common in compli-cated UTI, particularly in catheter-associated urinary tract infections (CAUTIs)34.
P mirabilis can cause urinary stones and crystalline biofilm formation associated with the increase of urine pH, due to urease production. Environmental changes caused by hydrolyzation of the urine urea allow the precipi-tation of minerals, promoting the formation of typical mineral-encrusted biofilms23. Due to encrustation, these crystalline biofilms may block the catheters in patients with CAUTIs. Sixty-two percent (62%) of patients with recurrent P mirabilis catheter encrustation developed bladder stones because of catheter colonization25. Other possible conse-quences associated with crystalline biofilms and blockages of the urinary catheter are urine retention, along with bladder and urethra mucosal trauma17.
Different factors participate in biofilm development, including motility, which has often been considered a puta-tive factor in this process21.
P mirabilis typical swarming motility is produced when vegetative swimmer cells differentiate into elongated, multinucleated and highly flagellated swarmer cells1. This process, mediated by flagella, is initiated by bacterial contact with a solid surface and probably contributes to biofilm formation and catheter colonization. The role of flagella and swarming in P mirabilis UTI is still under debate3,24. Different studies have suggested that flagella significantly contribute to P mirabilis urovirulence22,24. However, several works indicate that flagella are not critical for establishing UTI by P. mirabilis and other uropathogens18,19,35.
The role of flagella in P mirabilis biofilm formation remains unclear and is seldomly addressed in the lit-erature. A few studies have proposed that flagella and motility may play a role in the formation of biofilms9, while others consider flagella not to be critical in this process16.
In this study, we assess the input of P mirabilis flagella in biofilm formation using a flagellate mutant generated by allelic replacement, tested in different in vitro assays.
Materials and methods
Bacterial strainsThe clinical wild-type P mirabilis Pr2921 strain was exten-sively characterized36. An isogenic non-flagellate allelic replacement mutant (AF strain) was used to evaluate the role of flagella in biofilm formation. This mutant has both flaA and flaB structural genes partially deleted and interrupted by a Kanamycin cassette26, following the procedure used to generate other flagellate mutants in our laboratory19. Absence of swimming and swarming motility was evaluated, and the lack of flagella was evidenced by Western blot28. Bacteria were cultured in Luria-Bertani (LB) broth or LB agar (1.5%). Artificial urine (AU) was prepared according to Scavone et al.27 Growth curves were first assessed in LB and AU to check that the absence of flagella did not affect normal growth.
Bacterial suspensions were prepared in phosphate-buffered saline (PBS) from fresh LB agar plates at an OD 600 nm to inoculate LB or AU cultures in 96-multiwell plates27. pH of AU cultures of both strains was controlled serially.
Hydrophobicity of planktonic cellsTen microliters of bacterial suspensions in PBS (OD540 =0.2-0.3) were inoculated in 5ml of AU or LB, and incubated at 37°C for 48 h. Then, bacterial suspensions (OD600 =0.8) and 200 µl of added xylene were mixed, shaken for 2 min and left static for 20min. Subsequently, the aque-ous phase (OD600nm) was measured and hydrophobicity was assessed according to Bibiloni et al.2. The assay was done in triplicate. Hydrophobicity of planktonic cells of the different strains and under different conditions was assessed using the Tukey’s multiple comparison test.
Swimming and swarming motilitySwimming motility was evaluated as described by Hola et al.11, and swarming motility was assessed as described by Jones et al.15. Both assays were performed in triplicate.
Inhibition of swarming was evaluated using a specific P mirabilis flagella rabbit anti-serum. For this purpose, a rab-bit was immunized with four doses of 25 ^g of purified Pr2921 flagellin administered subcutaneously, using complete Fre-und’s adjuvant (Sigma) for the first dose and incomplete Freund’s adjuvant for the following ones, according to pre-vious studies19. These procedures were approved by the Institutional Animal Care Committee and adequate mea-sures were taken to minimize discomfort or stress of the animal. The antibody response was determined ten days after the last dose (day 55) and serum anti-flagella IgG titer measured by ELISA reached a value of 1:100000.
For the swarming inhibition assay, the WT strain was grown on 50 mm diameter plates containing 5 ml of modified LB agar and LB agar supplemented with the specific rabbit anti-serum, using a 1:10 dilution.
Bacterial migration across urethral cathetersThe ability to migrate across urinary catheters sections was assessed following the procedure proposed by Stickler and Huges32. The catheter materials were silicone and latex (Teleflex Medical, Kernen, Germany). Migration was tested 15 times for both catheter types.
Quantification of biofilm formation on polystyrene multiwell platesThe effect of the flagellar mutation in biofilm formation was evaluated using the semi-quantification technique based on the adsorption of crystal violet (CV)6.
A competition assay was also performed to assess the ability of both strains to remain attached to the polystyrene surface, forming a biofilm when cultured simultaneously following the procedure described above. However, in this case, the biofilms were scrapped and bacteria were cul-tured in nutrient agar and nutrient agar supplemented with kanamycin since AF carries a kanamycin-resistance gene incorporated into the mutagenic process26. Biofilm formation of the different strains and under different conditions was assessed using the Tukey’s multiple comparison test.
Biofilm formation over time by confocal laser scanning microscopyBiofilm formation of Pr2921 and AF was evaluated in 2, 5 and 7 day-LB and AU cultures under static conditions at 37°C27. This method allows biofilm formation on a cover-slip placed into a tube subjected to fluorescent staining after different incubation periods. Staining techniques and microscopy analyses were performed according to Schlapp et al.31. Bacteria were stained with Syto 9 (Molecular Probes) and the extracellular matrix with FilmTracer™ SYPRO® Ruby Biofilm matrix stain. 3D images were acquired using a Leica TCS LSI super-zoom confocal fluorescent microscope with an iXon Ultra 897 back-illuminated imaging EMCCD camera, LAS Software, a 100x oil immersion objective (NA=1.35) and 488/520, 450/610nm excitation/emission wavelength.
Three z-stacks were randomly chosen in each sam-ple, using an acquisition step of 0.3 ^m in the z-axis and 1024 x 1024 pixels in the xy-plane with a pixel size of 170 nm. After deconvolution using Huygens Software, segmentation, visualization, 3D reconstruction and biofilm parameter descriptors were calculated using ScianSoft31.
Bacterial number, bacterial and matrix volumes were calculated to compare biofilm evolution. Results were compared using one-way ANOVA p < 0.05.
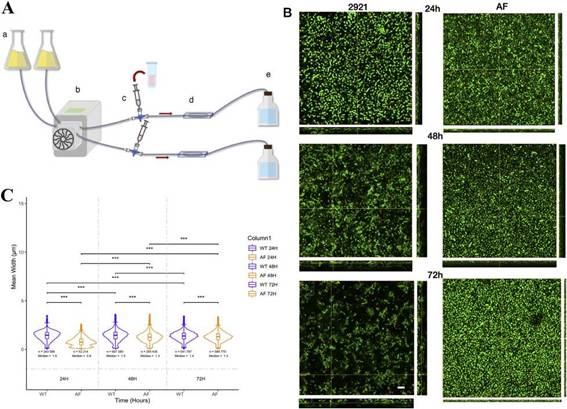
Biofilm grown under a flow systemIn order to evaluate biofilm formation under a flow system, we have developed a system that consists of a medium reser-voir, a peristaltic pump, a chamber where the biofilm was grown and a disposal flask. The chamber had two slides (bot-tom and top) in order to follow the biofilm formation by confocal microscopy. The system was set up with a LB flow at 0.5ml/min. First, the system was assembled and after removing air bubbles, the bacterial suspension was intro-duced into the chambers through a three-way stop cock. The biofilm was allowed to form for 1 h, after that the flow was started for 24, 48 and 72 h. Acridine orange (0.01 mg/ml) was used to stain and visualize bacteria in the biofilm. At different time points, the system was stopped and the dye was added to the system through the three-way stop cock. Visualization was performed in a LSM Zeiss 800, using a 63 x oil immersion objective (NA 1.4). Live images were taken every 127s for 3min. After acquisition, the system was loaded again until the next time point.
Image processing of time-lapse biofilmImage processing and analysis were performed using FIJI/ImageJ software30. Morphological analysis was performed using MicrobeJ plugin7. Morphological parameters were defined according to the different morphotypes of P mirabilis described by Jansen et al.14 Swarmer cells with a length greater than 10 ^m and a maximum width of 3 ^m were excluded from the analysis. Thresholds were used for binarization and length/width exclusion criteria prior to analysis. Contour detection was performed using Fit-Shape mode. The shape descriptors calculated from the data were length, mean width and aspect ratio, defined as the length of the major axis divided by the minor axis of the ellipsoid fitted to each particle. Statistical analysis was performed by RStudio software (RStudio Team, 2020). The differences between each time-lapse were analyzed with the Wilcoxon test. Motility analyses were performed with TrackMate plugin8,33. Motility analysis was performed using previously segmented time-lapse image sequences by Ilastik software. TrackMate auto intensity threshold tool was used to select all particles for track analysis. The trajec-tories of each individual bacterium were determined by LAP Tracker. It was set allowing a frame-to-frame linking and gap closing of 6 ^m, splitting and merging was set at 0.6 ^m, and ellipse long axis, ellipse short axis and max intensity were used as penalties for linking cost calculation. Mean square displacements (MSD) were calculated with R Studio software by Eq. (1) (RStudio Team, 2020). RStudio:
Figure 1: Growth curves of Pr2921 P. mirabilis wild-type strain and flagellate mutant AF in LB broth (LB) and artificial urine (AU). Growth curves were generated by serial OD measures of grown bacteria.
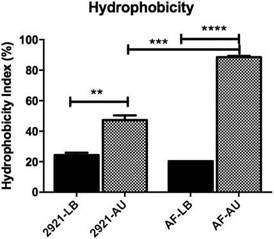
Figure 2: Hydrophobicity of Pr2921 P. mirabilis wild-type strain and flagellate mutant AF grown in LB broth (LB) and artificial urine (AU). When bacteria were grown in AU, the AF mutant exhibited a significant hydrophobicity increase compared with the wild-type Pr2921 strain inAU (p <0.001). Hydrophobicity was significantly higher in AU than in LB in the case of both strains (p <0.05). Sta-tistical differences were calculated using the Tukey’s multiple comparison test, **p <0.005, ***p <0.0005, ****p <0.0001.
Integrated Development for R. RStudio, PBC, Boston, MA (URL http://www.rstudio.com).
Results
Lack of flagella did not affect bacterial features, except flagellin expression
Growth curves of Pr2921 and AF cultured in LB or AU were similar, showing that the general biology of the mutant was not affected, and both strains grew better in LB compared with AU (Fig. 1).
pH of Pr2921 and AF cultures in AU reached a value of 9 after 6 h of incubation in both cases, remaining constant for at least 24 h, demonstrating that urease production was similar in both strains. Expression of MR/P fimbriae assessed by Western blotting (Suppl. Fig. S1), hemagglutination basedon MR/P fimbrial expression, and hemolysis observed on blood agar plates were similar in both strains (data not shown).
Genetic rearrangements due to allelic replacement mutagenesis were as expected and the mutant was unable to express flagellin28.
Hydrophobicity index is increased in AUWhen bacteria were grown in LB, no significant differ-ences were observed among the hydrophobicity indexes between both strains. However, when bacteria were grown in AU, the AF mutant exhibited a significant hydrophobic-ity increase compared with the wild-type Pr2921, in AU (p < 0.001) (Fig. 2). In addition, hydrophobicity of planktonic bacteria grown in AU was significantly higher than bacteria grown in LB, in the case of both strains (p < 0.05, Fig. 2).
Swimming and swarming motility and migration across latex and silicone catheter bridgesThe flagellate mutant was unable to swim or swarm (Suppl. Fig. S2). In both assays, the mutant grew to form a defined colony without spreading beyond the colony limits.
Swarming was completely inhibited when the WT strain (Pr2921) grew on LB agar supplemented with specific anti-flagella rabbit serum (1:10). Lower serum concentrations obtained by serial dilutions inhibited swarming in a dose-dependent relationship (data not shown).
The influence of flagella on P. mirabilis migration across catheter sections was assessed. The WT strain crossed latex and silicone catheter bridges in every case (15/15), while the AF mutant was unable to cross any catheter sections (0/15) (Suppl. Fig. S2).
Biofilm formation on polystyreneWhen biofilm formation was evaluated using the method based on associated CV adsorption, the AF mutant showed a significantly impaired ability to form biofilms compared to WT Pr2921 in LB (p <0.0001).
When the assay was performed using AU, the level of biofilm formation of the mutant strain was lower than that formed by the WT strain but the difference was not significant (p>0.05) (Fig. 3). A significantly reduced biofilm was also observed when WT grew in AU compared with that observed in LB (p <0.0001).
A competition assay was also performed to evaluate if the mutant was outcompeted by the WT strain in the biofilm. Results indicated that the wild-type strain outcompeted the mutant taking into account viable bacterial counts of Pr2921/AF from co-cultured biofilms. At 24h, wild type and
Figure 3: Biofilm formation on polystyrene surfaces assessed by the semi-quantitative method based on crystal violet staining.
Pr2921 P. mirabilis wild-type strain and flagellate mutant AF were grown in LB broth (LB) and in artificial urine (AU) and incu-bated at 37°C for 48 h. Box plot of the OD values obtained in each condition are shown; asterisks show significant differences among different conditions. Statistical differences were calcu-lated using the Tukey’s multiple comparison test, **p <0.005, ****p <0.0001. utant counts (log) were 7.63 ±0.01 and 2.28 ±0.2, respec-tively (p<0.05). After 48h, wild type and mutant counts remained at similar levels (7.28 ±0.07 and 2.64 ±0.09, respectively, p< 0.05).
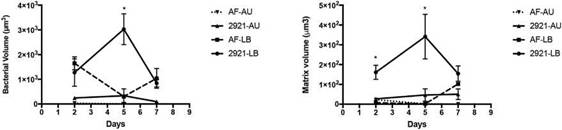
Biofilm formation dynamicsBiofilm formation was evaluated in LB and AU under static conditions at 37°C, at 2, 5 and 7 days of incubation, using CLSM and image analysis (Figs. 4 and 5).
When biofilm formation was assessed after 2 days in LB or AU, the bacterial volume and the extracellular matrix of WT and the flagellate mutant AF were similar, showing in general low values with no significant differences among strains or media conditions (p > 0.05) (Fig. 4). However, after 5 days, significant differences were observed among WT and the mutant AF strains grown in LB. The WT bacterial and matrix volumes significantly increased compared to the AF mutant (p<0.0001 in both cases). When both strains were cultured in AU, no AF bacteria remained attached to the coverslip, while WT Pr2921 exhibited a structured biofilm. However, the statistical difference was not significant since the WT biofilm was small. Furthermore, the AF matrix in AU was almost inexistent.
After 7 days of incubation in LB, the bacterial and matrix volume of the biofilm formed by WT 2921 decreased and dif-ferences were not significant when compared to the biofilm formed by AF (p>0.05). When bacteria were cultured in
AU, no statistical differences between strains were iden-tified; however, a small WT 2921 biofilm (both bacteria and matrix) was still observed, but not with the AF mutant (Figs. 4 and 5).
The dynamic system allowed in vivo observation of the biofilm every 24 h (Fig. 6A). Both strains formed biofilm, but differences were observed between WT and the AF mutant. At 24 h, biofilms were flat as observed by the height in the 3D stacks (Fig. 6B upper panels). WT 2921 biofilm was around 1.25 ^m in height while the AF biofilm was 0.75 ^m in height. Moreover, significant differences were observed in the size and shape of the bacteria (Fig. 6B). WT 2921 was more elon-gated than AF as represented by the mean width (the length of the major axis divided by the minor axis of the ellipsoid fitted to each bacteria) (Figs. 6B and C). At 48 h, WT 2921 increased the 3D biofilm structure reaching 2 ^m in height and forming channels while the AF biofilm remained flat. At 72 h, AF showed a compacted and thin biofilm with short-ened bacteria and WT 2921 had a heterogeneous bacterial morphology within a 3D biofilm with the presence of channels (Fig. 6B, Suppl. MSD). While WT 2921 formed a mature biofilm with different bacterial morphologies, the AF mutant strain showed a flat biofilm and the morphology of the bacteria was reduced over time. With regard to the length of the bacteria, we observed that 2921 acquired a more elongated morphology while AF was shortened (Figs. 6B and C).
Discussion
P. mirabilis is among the most frequent causes of compli-cated UTI28, and displays a broad and complex range of virulence factors that may participate in the colonization of different surfaces12,27.
Flagella have been associated with the formation of biofilms in different pathogens, including Bacillus cereus, Yersinia enterocolitica and Helicobacter pylori10,20. However, the role of flagella and swarming motility in P. mirabilis UTI and biofilm formation has been thoroughly debated19,22,29.
In this study, we used a flagellate mutant to assess the role of flagella in P mirabilis biofilm formation, through different experimental in vitro approaches, which are dis-cussed below.
Growth patterns were similar when WT Pr2921 and the AF mutant were grown in LB broth or AU, indicating that the mutation did not alter the general bacterial biology. In addition, a series of phenotypic pathogenic features were not altered by the mutagenesis process, including ure-ase production, fimbriae expression, hemagglutination and hemolysis production.
The influence of flagella on R mirabilis surface-related features was assessed by measuring bacterial hydropho-bicity. According to different authors, bacterial surface hydrophobicity is important for adherence to different interfaces and biofilm formation4. When the flagellate mutant was grown in LB broth, bacterial hydrophobicity did not vary. However, when grown in AU, hydrophobicity significantly increased, probably associated with an increased exposure of the lipid components of the bacterial surface. In other studies, no clear relationships between bacterial hydrophobicity and enhancement of binding to different substrates were observed5.
Figure 4: Bacteria and matrix volumes in biofilms formed by Pr2921 P. mirabilis wild-type strain and flagellate mutant AF in LB broth (LB) and artificial urine (AU) over time. Total bacteria and matrix volumes in biofilms formed in LB broth (LB) and artificial urine (AU) are shown in panels (A) and (B), respectively. *p <0.05. The symbols represent the means of 3-5 random fields and their corresponding standard errors.
Figure 5: 3D reconstruction of biofilms formed by Pr2921 P. mirabilis wild-type strain and flagellate mutant AF in 7 day-cultures in LB broth (LB) and artificial urine (AU). All images were taken using the same magnification (63x oil immersion objective). Bacteria are represented in green and the matrix in red. D2: two day-cultures; D5: five day-cultures; D7: seven day-cultures.
In this study, we also assessed the ability of the iso-genic flagellate mutant to swim, swarm and migrate over catheter sections of different materials. The mutant was unable to swim or swarm on LB agar or to migrate along sections of latex and silicone catheters, strongly confirm-ing the role of flagella in the colonization of these clinical devices. These results are in accordance with previous studies15.
Figure 6: Time-lapse biofilms. (A) The dynamic flow system that consists of a medium reservoir (a), a peristaltic pump (b), the 3-way stop cock (c), the biofilm chamber (d) and the disposal flask (e). (B) Representative 3D stacks of each time point (24, 48 and 72 h) of Pr2921 (left) and AF (right). The scale bar represents 10 ^m, and applies to all images. (C) Aspect ratio, defined as the length of the major axis divided into the minor axis of the ellipsoid that is fitted to each particle. Significant differences were calculated using the *** P<0.05.
The inclusion of specific P mirabilis antiflagella antibod-ies in the culture medium also resulted in the abolition of motility, reinforcing the clear and specific role of flagellar function in motility and spreading over different surfaces through an indirect approach.
When biofilm formation was assessed on an abiotic sur-face like polystyrene, the flagellate mutant grown in LB showed a significant smaller biofilm compared with the wild type Pr2921. When bacteria grew in AU, the mutant also formed a smaller biofilm although differences were not significant. In this case, it must be taken into account that P. mirabilis growth in AU was significantly less abundant than in LB broth, influencing the magnitude of growth differences in both strains.
A competition assay based on the wild type and the mutant co-cultivation was performed to refine the assessment of flagella contribution in biofilm formation. We had used this kind of approach before to detect subtle effects of different P mirabilis virulence factors in ascending UTI in mice13. Results of this assay revealed that although the mutant was outcompeted by the WT strain, a biofilm was formed that even persisted for at least 48 h, indicating that biofilm formation was not completely abolished by the lack of flagella.
When CLSM was used to assess biofilm formation in LB broth and AU, significant differences were observed among biofilm parameters at different stages. One of the most notorious differences was the impaired capacity of the AF mutant to grow and produce extracellular matrix at the biofilm maturation stage in the static model (5 days). At this stage, the AF mutant biofilm had almost disap-peared in AU while the WT biofilm, although small, remained attached and showing a typical mature structure, remaining attached even after 7 days. Our results confirm that flagella are important for biofilm formation, under different con-ditions. Furthermore, differences in bacterial morphology were observed under dynamic conditions, but the AF mutant was able to form a biofilm.
Different authors have reported that mutations of P. mirabilis virulence genes have different effects on biofilm formation that can induce a decrease or even an increase in matrix or bacterial biovolumes12,36.
Several studies have reported the role of bacterial flagella in adhesion to biotic and abiotic surfaces, including P. mirabilis, which can contribute to the initial contact with different surfaces and even among cells15,35.
As mentioned above, Jones et al.16 suggested that nei-ther swarming nor swimming motility are required for the attachment of P mirabilis to catheters and that mutants unable to swarm and swim formed crystalline biofilms and blocked catheters even more rapidly than the wild-type strain. The authors hypothesize that on contact with a sur-face, the non-swarmer cells are more likely to remain at the site of attachment, divide or produce microcolonies that initiate biofilm formation. On the other hand, Fusco et al.9 compared different P. mirabilis strains and reported that motility ability was related to biofilm formation; however, they did not use isogenic mutants.
Conclusión
Overall, our results show that flagella critically contribute to the formation of P. mirabilis biofilms. However, the lack of this organelle could not completely prevent biofilm formation, suggesting that different elements drive biofilm formation and persistence. Like other factors (e.g., ATF fimbria)36, flagella could play a significant role in biofilm formation on abiotic surfaces rather than in urinary tract colonization and infection.
This knowledge could contribute to the design of strate-gies for P. mirabilis biofilm prevention targeting the flagellar function, possibly in combination with other specific mechanisms.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This research was supported by grant FCE 4760 awarded by ANII-Uruguay and PEDECIBA-Uruguay. JJW is supported by FONDECYT 3220832. SH is supported by the Chilean Millen-nium Science Initiative P09-015-F, FONDECYT 1181823, DAAD 57519605, MINEDUC RED 21994, CORFO 16CTTS-66390 and AUCI Program for South-South Collaboration Uruguay-Chile, ACM 170003.












 uBio
uBio