Introduction
Amphibians have a significant role as a study model in physiology, and this group is a standard model for the study of many biological processes. However, relatively few studies have been carried out on the amphibian gastrointestinal tract16. Some reports are investigating the gastrointestinal epithelial transformation from the larval to adult type and apoptosis (21,22). Additionally, histological and histochemical changes in the digestive tract of Ceratophrys ornata and Xenopus laevis during metamorphic climax were reported18.
In general, studies related to the digestive system were carried out on reptiles, birds, and mammals. But the cell renewal system of the adult epithelium in the amphibian stomach is similar to the mammalian stomach, where cell proliferation is localized in the neck region of gastric glands. Therefore, the amphibian digestive tract serves as a model system for studying mammalian organ regeneration. Nonetheless, there are few studies about the structure of the gastrointestinal tract in amphibians 1), (2), (8), (16), (20), (25), (27), (31), (34).
The genus Melanophryniscus, is distributed from the center and southeast of Brazil, south of Bolivia, Paraguay, Uruguay, and the north and center of Argentina (Bol. Asoc. Herpet 12: 71-76) to the savannas of Buenos Aires23. There are brief descriptions of the diets of Melanophryniscus moreirae and M. stelzneri. There is also a description of the diet of M. montevidensis32, a Uruguayan species of the genus, that relates the ant content in the diet to the production of skin alkaloids9 .
Some authors described the diet of three populations of M. rubriventris (4) in Argentina. Regionally, de- scribed and analyzed the diet ofM. cupreuscapularis in comparison to other species(15,16). For M. klappenbachi, there is knowledge about the main alkaloids present in the species skin and a general description of its diet11.
Due to the lack of studies on the digestive system of anurans, particularly those that relate its structure with dietary habits, this work aimed to study the diet and the histomorphological structure of the gastrointestinal tract of M. klappenbachi with the hope of contributing to the natural history of anurans in general and specially bufonids of this genus.
Materials and methods
Collection
We collected 29 adult and juvenile specimens of M. klappenbachi of March a June 2015 in a private field located 20 km from the entrance of the “Las Palmas” locality, in Bermejo department, Chaco province, Argentina. We added the specimens to the Herpetological Collection of the National University of the Northeast (UNNEC), numbered as follows: UNNEC 13081 a 13109.
The site is characterized by remnants of Spinello and algarrobillo woodlands associated with palm trees and grasslands with flood zones, typical of Oriental Semihumid Chaco. The field is near the Paraná River, surrounded by a degraded forest.
The methodology consisted of “Surveys at breeding sites”32, with monthly surveys carried out between March and June 2015. We took the samples manually, without distinction of sex or size. We transferred the specimens to the laboratory in plastic bags and dampened containers.
We euthanized the specimens using benzocaine hydrochloride 250 mg/l, standard IACUC protocol, and CICUAL Fmed-UNNE 004/18. Then, we fixated the specimens with Bouin solution and preserved them in 10% formaldehyde. We measured each specimen with a digital caliper (0.01 mm accuracy); the measures taken were: snout-vent length (SVL) and mouth width (MW).
Anatomical analysis
We performed an incision on the ventral region and extracted the complete digestive tract. We measured the tract using a digital caliper (0.01 mm accuracy). The measures taken were the width and length of the stomach and intestines and the total length of the digestive tract (TLD).
Later, we fixated the tracts with Bouin solution and preserved them in 10% buffered formaldehyde. Then, we dehydrated the samples, included them in paraffin, cut them in 6 µm sections and stained them with hematoxylin and eosin (HE), periodic acid-Schiff (PAS) and Gómóri trichrome stains. We observed and photographed the samples with a Leica trinocular microscope (DME model). We determined the sex of each specimen by direct observation of the gonads. Then, we analyzed the digestive content under a stereomicroscope.
We dissected the specimens and cut their digestive tracts for observation under a Scanning Electron Microscope (SEM). We dehydrated, dried, and metalized the sections with gold.
We carried out the observations with a JEOL JSM-5800 LV microscope belonging to the Scanning Electron Microscopy Service of the General Secretariat of Science and Technology (SGCyT) of the National University of the Northeast (UNNE).
Data analysis
We identified each prey item to the lowest possible taxonomic level. We calculated the trophic diversity of the species using the Shannon-Weaver diversity index33. We compared the size of the specimens in terms of SVL, MW, and TLD between males and females using the Mann-Whitney U Test. We determined the mean number of prey per stomach for the complete sample and each sex.
We determined the Spearman (r) correlation coefficient for SVL-MW, SVL-TLD, and SVL-number of prey per stomach. We carried out every analysis using the statistical analysis programs Past19 and Infostat12 and the spreadsheet Microsoft Excel.
Results
Diet
We captured a total of 29 specimens of March a June 2015. We only considered adult specimens (19 females, 8 males) with identifiable stomach or intestine content. In the case of juvenile specimens, only their histomorphology was taken into account since they did not present gastro-intestinal content at the time of capture.
The diet consisted of 737 items classified into 6 prey categories (Table 1) and was numerically dominated by ants. Additionally, ants were the most frequent prey category, found in all analyzed specimens (100 %). The mean number of prey per stomach was 27 (± 25), 26 (± 24) for males, and 29 (± 27) for females. We did not observe significant differences in the number of prey per stomach between sexes (Mann-Whitney U
Test = 109.00; n = 27; P = 0.95).
The trophic diversity was 0.43, according to the Shannon-Weaver index (0.48 for females, 0.24 for males). We found no significant differences between the diets of males and females of this species (Mann- Whitney U Test = 97.00; n = 27; P = 0.47).
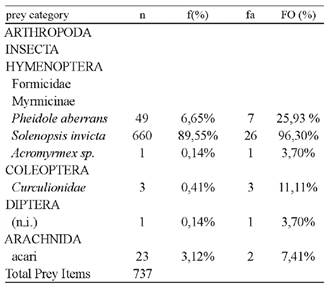
Table l Types of prey found in the gastrointestinal tracts of 29 specimens of Melanophryniscus klappenbachi capture on March to June 2015 in Bermejo, Chaco, Argentina. n = number of prey per category, f (%) = percentage of each category in the overall sample, fa = number of gastrointestinal tracts with each category, and FO (%) = frequency of occurrence.
Gastrointestinal morphometry
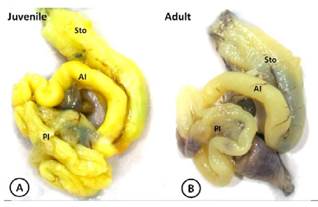
The gastrointestinal tract of M. klappenbachi was divided into three main regions: stomach, small intestine, and large intestine (Figure 1). The mean MW was 7.8 mm (± 0.51) for males (n = 8) and 8.08 mm (± 0.54) for females (n = 19). The mean SVL was 28.34 mm (± 1.3) for males and 29.52 mm (± 1.88) for females.
The mean TDL was 62.83 mm (± 8.92), 61.32 mm (± 5.29) for males, and 64.95 mm (± 8.40) for females. We did not find significant differences in SVL (Mann-Whitney U Test = 74.00; n = 27; P = 0.08), MW (Mann-Whitney U Test = 77.00; n = 27; P = 0.11) or TDL (Mann-Whitney U Test = 89.00; n = 27; P = 0.38) between males and females. We found a positive and significant correlation between the SVL (r = 0.56) and the MW (r = 0.56), with P = 0.002 and n = 27. We also found a positive and significant correlation between SVL (r = 0.64) and TLD (r = 0.64), with P = 0.0004 and n = 27. We found no association between SVL and the number of prey per stomach (r = 0.013), with P = 0.95 and n = 27.
Histomorphology
The histological structure of the digestive tract in both juveniles (Fig. 2, 4 and 5) and adults (Figs. 3 and 6) is composed of the typical layers observed in vertebrates: mucosa, submucosa, external muscular and serous (Figs. 2 A and 3 A).
Stomach
In the stomach, juveniles, and adults, the mucosa had gastric folds and a simple layer of epithelium lining composed of mucous cells in the spine. It has gastric glands located at the base of the barrel-shaped grooves called gastric fossae (Fig. 2 A and 3 B). The apical domain of epithelial cells was found to be acidophilous and exhibited a strong positive reaction for PAS (Fig. 2 D, 3C).
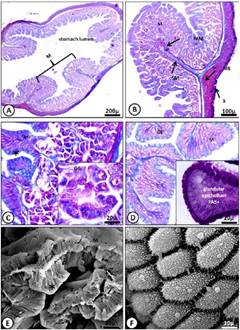
Figure 2 Micrograph of the juvenile stomach of M. klappenbachi. A) Layers of the stomach; M, mucosa; MM, muscularis mucosae; SM, submucosa; ME, muscular; S, serosa. Gomori trichrome stain. B) Mucosa. GE, glandular epithelium; GG, gastric glands. Gomori trichrome stain. Cells and gastric glands. C) Glandular epithelium (*) and gastric glands. Gomori trichrome stain. D) Positive PAS reaction for apical portions of mucous. E-F) SEM microphotographs showing in detail the gastric folds (E) and mucosal epithelial cells (F).
The mucosa is separated from the submucosa by a muscular layer of the mucosa. The gastric glands were structured as simple tubular glands, often branched to the lower portion of the gland and composed of mucous cells of the neck, located mainly in the neck region (Fig. 2 C, 3 B and D). Through SEM, it was possible to observe the gastric ostioles. Superficial mucous cells, such as neck cells, showed bright purple staining after treatment with PAS, indicating the presence of glyco-proteins.
The submucosa was formed by loose connective tissue rich in blood vessels, as evidenced by strong staining after treatment with Gómóri trichrome staining; however, we did not find glands in this region. The external muscle was also made of smooth muscle tissue. The serosa was observed as a thin layer of loose connective tissue with some blood vessels, covered with mesothelium.
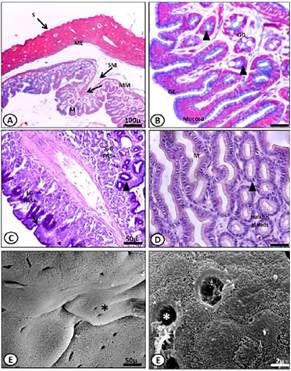
Figure 3 Micrograph of the stomach adult of M. klappenbachi. A) Layers of the stomach; M, mucosa; MM, muscularis mucosa; SM, submucosa; ME, muscular, S, serosa. Gomori trichrome stain. B) Mucosa. GE, glandular epithelium; GG, gastric glands. Gomori trichrome stain. Cells and gastric glands. C) Positive PAS reaction for apical portions of mucous. D) Glandular epithelium and gastric glands. HE stains. E-F) SEM microphotographs showing in detail the gastric folds (E) and mucosal epithelial cells and ostioles (F).
Intestine
The mucosa of the intestine (thin and thick) of both juveniles and adults showed several high longitudinal folds called intestinal villi (Fig. 5 E). These villi were composed of columnar enterocytes with microvilli on their apical surface (Fig. 4 B, 5 E). We also found a large number of goblet cells in this epithelium.
These cells showed strong staining after treatment with PAS (Fig. 4 and 6 D), indicating the presence of glycoproteins, a product of the secretion of these unicellular glands. The submucosa was composed of loose connective tissue with some vascularization. The muscle showed moderate development in the sections. The outermost layer, the serosa, was composed of a thin layer of connective tissue.
The difference between juveniles and adults in the intestine was given at the level of abundance of goblet cells, the number in the region of the large intestine of the juveniles being greater, while in adults, that difference was not evident (Figure 4 and 6).
Discussion
The analysis carried out in this study revealed that in M. klappenbachi, the preponderance of hymenopterans of the family Formicidae is significant, constituting more than 95% of the prey sample. These ants belonged to three species of the subfamily Myrmicinae. These taxa were followed in abundance by mites.
Analyzed feces from M. montevidensis, revealing the presence of ants and mites32. Observed the preponderance of ants in the diets of M. cupreuscapularis and M. klappenbachi10. Some authors described and analyzed the diet of M. cupreuscapularis, comparing it with other bufonids 14,15. Finally, in 2013 carried out a study about the diet of M. devincenzii, also showing the predominance of mites followed by ants5.
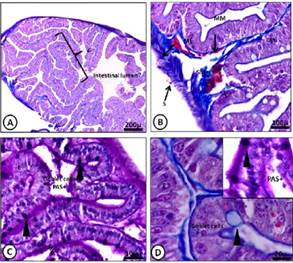
Figure 4 A-D) Light micrograph of the anterior intes tine of M. klappenbachi (juvenile). A) Layers of the intestine; M, mucosa; SM, submucosa; ME, muscular, S, serosa, Mucosa folds or villi (V). Gomori trichro- me stain. B) M, mucosa; SM, submucosa; L, lacteal. Gomori trichrome stain C) Intestine layers and com- ponents; MV, microvilli; E, enterocytes; Goblet cells positive PAS. PAS stain. D) Positive PAS reaction for globlet cells. Gomori trichrome and PAS stain.
Taking into account numerical frequencies, we consider M. klappenbachi an ant specialist. Matching the results from numerous previous studies that maintain that specialist foragers use active search for the capture of their prey and prefer small and hard prey like ants and beetles24, we consider M. klappenbachi an active predator since these toads pursue their prey and demonstrate a clear tendency towards myrmecophagy.
In addition to the analyzed prey, there was also plant material found in the alimentary tracts, but this was not included in the study since its ingestion was deemed accidental. Observed that a certain amount of the stomach contents of Bombina variegate (29.82%) consisted of plant fragments and suggested, too, that they were eaten by accident19.
The same accidental ingestion was postulated for M. devincenzii5 . Some authors considered that the ingestion of plant fragments is helpful for anurans in terms of gut parasites elimination and nutritional and even hydric contribution3.
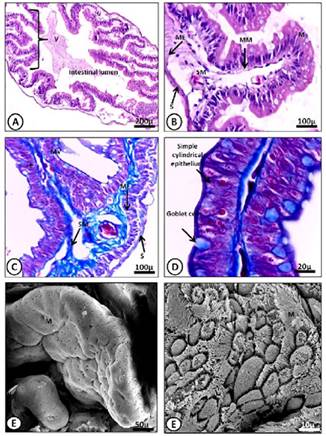
Figure 5 A-D) Light micrograph of the posterior intestine of M. klappenbachi (juvenile). A) Layers of the intestine; M, mucosa; SM, submucosa; ME, muscular, S, serosa, Mucosa folds, or villi (V). HE stains. B) Detail of Mucosa fold. M, mucosa; SM, submucosa. HE stains C) Intestine layers and components; MV, microvilli; E, enterocytes; Globet cells. Gomori trichrome stain. D) Detail goblet cells and enterocytes Gomori trichrome stain. E-F) SEM microphotographs showing in detail the intestinal folds (E) and mucosal epithelial cells and microvilli (F).
Studies regarding the intersexual differences in the diets of anurans are scarce due to the difficulty of capturing females of many species27. This variation between sexes could reflect the different requirements of each sex(13,29) or different foraging strategies6 since males need more energy for vocalization and territorial defense, and females need energy for egg production during the breeding season 30. In the present work a significant difference between both sexes could not be found.
The gastrointestinal tract showed the typical structure observed in vertebrates; in the same way, the typical cell and mucosa arrangements were observed. Some authors have presented similar results in Rhinella icterica28 and Hyla orientalis1.
These results provide a basis for the analysis of the gastrointestinal histomorphology and leave an open path to a more specific study regarding each region of the digestive system.
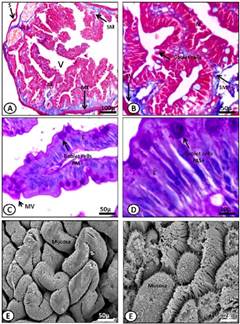
Figure 6 A-D) Light micrograph of the anterior intestine (A, B) and posterior intestine (C, D) of M. klappenbachi (adult). A) Layers of the intestine; M, mucosa; SM, submucosa; ME, muscular, S, serosa, Mucosa folds, or villi (V). Gomori trichrome stain. B) Detail of Mucosa fold. M, mucosa; SM, submucosa. Gomori trichrome stain. C) Details fold intestinal; MV, microvilli; E, enterocytes; Goblet cells. PAS stain. D) Detail goblet cells and enterocytes. PAS stain. E-F) SEM microphotographs showing in detail the intestinal folds (E) and mucosal epithelial cells and microvilli (F).
The PAS staining was positive for the gastric glands and apical portions of the mucous stomach cells, and the goblet cells present in the intestines, due to the presence of glycoproteins. These results concur with previous observations in other amphibians, Triturus carnifex27, Bufo viridis26, Hyla orientalis1, Rana aurora17) and in some fish12 reptiles7 and mammals35.
These glycoconjugates are of great importance since they possess a significant role that includes maintaining the moisture of the tissues, lubrication, and reduction of mechanical friction, including the friction produced by feces when leaving the alimentary canal1, and the protection against chemicals and pathogens.
We consider that the data provided by this work is of importance as a basis for future studies that include both the trophic analysis of M. klappenbachi and its role in the obtainment and presentation of skin alkaloids10, not only for this species but for other anurans of the genus Melanophryniscus as well.
This work also characterizes the general histological structure of the gastrointestinal tract of the species and aims to serve as a foundation for future studies, both comparative and specific.
Acknowledgments. To the research projects of the General Secretary of Science and Technology of the National University of the Northeast (SGCyT-UNNE) (PI 16F013: Natural History of amphibians and reptiles of north-eastern Argentina, Director: Céspedez, Jorge).