Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista argentina de cardiología
On-line version ISSN 1850-3748
Rev. argent. cardiol. vol.92 no.1 Ciudad Autónoma de Buenos Aires Mar. 2024 Epub Feb 28, 2024
http://dx.doi.org/10.7775/rac.es.v92.i1.20739
ORIGINAL ARTICLE
Eccentric Vascular Remodeling: Its Relationship with Metabolic Disorders and Increased Body Mass
1Hospital Universitario Austral. Centro Cardiometabólico Officia, Servicio de Cardiología, Buenos Aires, Argentina.
2Centro CardioArenales, Buenos Aires, Argentina.
Background:
Recent evidence would establish muscle hypoperfusion as the primary cause of metabolic disorders in response to overfeeding. This centripetal concept on the development of metabolic disorders could involve not only alterations in the microvasculature, but also affect the conductance arteries.
Objectives:
The aim of this study was 1) to determine the association between baseline brachial artery diameter (BAD) and flow-mediated vasodilation (FMVD), 2) To analyze the association of both parameters throughout the increase in body mass, 3) To evaluate associations between BAD/FMVD with components of the metabolic syndrome (MS) and 4) To evaluate the independent association of both variables with MS.
Methods:
A total of 3493 patients were evaluated. Patients <18 and >80 years old, those with previous cardiovascular disease, chronic kidney disease (CKD), collagenopathies, or treated with statins were excluded from the study. Blood pressure (BP), anthropometric parameters and metabolic profile were determined, and the subjects were classified according to the presence of MS conforming AHA/NHLBI 2019 criteria. BAD was measured in mm and FMVD as percentage. The linear association between BAD and FMVD was assessed, and both variables were analyzed according to deciles of body mass index (BMI). Associations between BAD/FMVD with BP, glucose (Glu), triglycerides (TG) and high-density cholesterol (HDL-C) levels were evaluated. Two logistic regression analyses were performed with MS as dependent variable and BAD or FMVD plus age, gender, BMI, and coronary risk factors (CRF) as independent variables.
Results:
A total of 1995 patients (48.2 ± 11 years, 56% men) were admitted in the study. An inverse correlation was found between BAD and FMVD (r= -0.42; p < 0.0001). BAD increased according to deciles of BMI (p < 0.000001), while FMVD showed an inverse relationship with increasing deciles of BMI (p < 0.000001). BAD exhibited a direct correlation with BP, Glu and TG; and an inverse relationship with HDL-C (p < 0.05 in all cases). FMVD presented an inverse correlation with BP, Glu and TG; and a direct correlation with HDL-C (p < 0.05 in all cases). BAD was independently associated with MS adjusted for age, gender, BMI and CRF (OR 1.42, p=0.0019), while FMVD was not (OR 0.98, p = 0.217).
Conclusion:
Eccentric vascular remodeling was associated with vascular adaptation to increased blood flow demand and with metabolic alterations throughout the increase in body mass. Thus, the dynamic compromise of vasculature could play a decisive role in the development of metabolic alterations occurring synchronously with weight gain.
Key words: Vascular remodeling; Metabolic syndrome; Obesity; Flow-mediated vasodilation
INTRODUCTION
The increase in body mass observed in overweight and obese subjects is associated with hemodynamic alterations, the most important being the increase in plasma volume and vascular dysfunction. From a pathophysiological point of view, different endocrine alterations act concurrently and synergistically in the development of these circulatory alterations.
With the increase in body mass - particularly in obese people - the increase in water and sodium reabsorption prompted by insulin through the activation of the sodium-hydrogen exchanger in the proximal convoluted tubule,1 hyperreninemia derived from increased sympathetic outflow, 2 decreased tubular flow rate and stimulation of tubuloglomerular feedback, 3 in addition to increased autonomous secretion of aldosterone in dysfunctional adipocytes 4 promotes a circulatory state characterized by increased cardiac output and eccentric remodeling of the cardiac chambers and conductance arteries, which coexists with enhanced tone of the precapillary sphincters and development of peripheral hypoperfusion. Since the delivery of macronutrients to metabolically active organs, such as skeletal muscle and adipose tissue, is largely dependent on adequate perfusion, the inability to increase blood flow during the postprandial period can be associated with metabolic alterations in overweight and obese subjects. As an example, blocking insulin-mediated muscle blood flow reduces glucose consumption in the muscle by 40% according to the euglycemic-hyperinsulinemic clamp technique. 5 In this scenario, the alterations in the structure and function of the conductance arteries that participate in muscle perfusion (which consume 75 to 80% of the total body glucose) 6 added to the increase in peripheral resistance disorders could jointly induce metabolic disorders as a peripheral starting point with the progressive increase in body mass.
METHODS
A total of 3493 patients from the metabolic database of the CARFARE registry (CARDIOMETABOLIC RISK FACTORS REGISTRY), carried out for a cardiovascular prevention program of the Officia Cardiometabolic Unit, from the Cardiology service of Hospital Universitario Austral from July 2016 to January 2020, were evaluated .These patients underwent a structured, sequential evaluation, on the same date, consisting of laboratory analyses that included urine metabolites and peripheral blood assessments after 12-hour fasting. Subsequently, medical interrogation with data collection on cardiovascular risk factors (CRF) and clinicalcardiological history, weight and height measurements with body mass index (BMI) calculation, baseline blood pressure (BP) (at rest, 3 determinations), and performance of different imaging studies were performed.
For practical purposes, data on the pathological history, CRF, metabolic profile measurements - glucose (Glu), HDL cholesterol (HDL-C) and triglyceride (TG) levels in peripheral blood and flow-mediated vasodilation (FMVD) were used. Obesity was defined according to the World Health Organization (WHO) as BMI ≥ 30 kg/m2, and metabolic syndrome (MS) according to the IDF Joint Interim Statement and 2019 AHA/NHLBI 7 as the presence of 3 of the following criteria: TG >150mg /dL, HDL < 40 mg/dL in men and < 50 mg/dL in women, BP >130/85 mm Hg, glucose >100 mg/ dL, and treatment for diabetes mellitus (DM), hypertension (HTN) or dyslipidemia.
A high-resolution vascular ultrasound machine (Phillips HD7 XE, Koninklijke Philips N.V) equipped with a 10 MHz linear array probe was used for FMVD. This procedure was performed on the brachial artery, in a quiet environment, at 22°C, with 12-hour fasting, without medication intake, during the morning, and in the absence of antihypertensive drugs for a period of 12 hours. The calculation was performed using the following formula: FMVD = [(baseline BAD in mm - post-ischemia BAD in mm) / baseline BAD in mm] × 100. Ischemia was induced through 30 mm Hg supra- systolic compression with a cuff placed on the brachial artery of the left arm, 3 to 5 cm above the elbow crease for 3 minutes. The data on baseline BAD and FMVD were collected in mm and percentage, respectively.
For the present analysis, patients aged < 18 and >80 years, those with a history of ischemic heart disease (chronic stable angina, unstable angina, acute myocardial infarction), heart failure, chronic arrhythmia or significant arrhythmic events, transient ischemic stroke, stroke or peripheral vascular disease, stage III or higher chronic kidney disease, known rheumatological diseases and decompensated chronic diseases, as well as incomplete data, were excluded. Patients treated with statins or β2 adrenergic agonists, due to their effect on endothelial function, were also excluded from the analysis.
A linear relationship between BAD and FMVD was analyzed, and each of these variables (BAD and FMVD) was subsequently evaluated according to BMI levels. Linear associations between BAD and FMVD with constituent variables of MS (BP, GLu, TG and HDL-C) were evaluated. Two logistic regression analyses were performed to explore the independent association of both variables with MS.
Statistical analysis
Baseline characteristics are expressed as mean and standard deviation for continuous variables, and as number of cases and percentage for categorical variables. Linear correlation is expressed with the Pearson r coefficient in the case of normally distributed variables and Spearman's rho in those with a non-normal distribution. BMI levels were obtained through stratification into deciles. The analysis of variables according to deciles was carried out using ANOVA in those with a normal distribution, and the Kruskall Wallis test in those with a non-normal distribution. Given the probable collinearity of BAD and FMVD, two logistic regressions were carried out: 1- with MS as the dependent variable and BAD, age, gender, BMI and CRF (dyslipidemia, HTN, DM, smoking, sedentary lifestyle) as independent variables, and 2- with MS as the dependent variable and FMVD, age, gender, BMI and CRF (dyslipidemia, HBP, DM, smoking, sedentary lifestyle) as independent variables. A value of p < 0.05 was considered statistically significant. The analysis was performed with MedCalc 20.2.17 statistical software package.
RESULTS
A total of 1995 patients were included in the study (48.2±11 years, 56% male, BMI 27.9±5.89 kg/m2, MS 20.3%). The population presented patients with low prevalence of smoking, dyslipidemia and DM (Table 1).
Tabla. 1 Características basales (n=1995)
| Variable | |
|---|---|
| Age, years (mean ± SD) | 48.2 ± 11.2 |
| Male gender (%) | 56 |
| BMI, kg/m2 (mean ± SD) | 27.9 ± 5.9 |
| HTN (%) | 26 |
| Smoking (%) | 14 |
| DM (%) | 4 |
| Dyslipidemia (%) | 25.9 |
| Sedentary lifestyle (%) | 33 |
DBT: diabetes; DE: desviación estándar; HTA: hipertensión arterial IMC: índice de masa corporal
In the univariate analysis, BAD and FMVD showed a significant inverse relationship (r: -0.42, p < 0.0001). BAD increased across BMI deciles (p < 0.000001) (Figure 1) and, conversely, FMVD presented a progressive reduction across BMI deciles (p < 0.000001) (Figure 2). A direct correlation was found between BAD and BP (r=0.26, p < 0.001), Glu (r=0.25, p < 0.001) and TG (r=0.26, p < 0.001) and an inverse correlation with HDL-C (r= - 0.35, p < 0.001). On the other hand, FMVD presented weaker inverse associations with BP (r= -0.15, p < 0.001), Glu (r= - 0.11, p < 0.001) and TG (r= -0.12, p < 0.001) and a direct correlation with HDL-C (r= 0.14, p < 0.001).
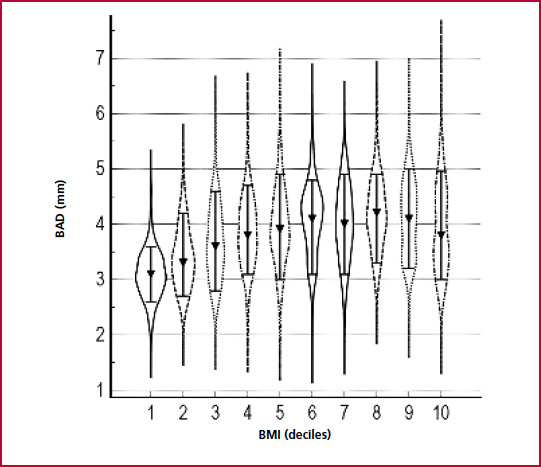
Fig. 1 Brachial artery diameter according to deciles of body mass index. Kruskall Wallis test, p < 0.000001. Values expressed as median (10-90% CI). BAD: Baseline brachial artery diameter. BMI: Body mass index
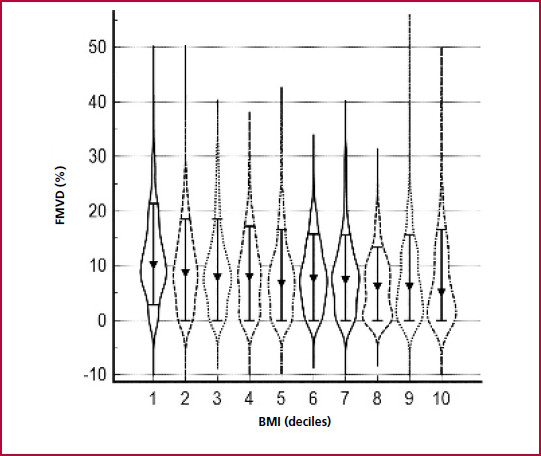
Fig. 2 Flow-mediated vasodilation according to deciles of body mass index. Kruskall Wallis test, p < 0.000001. Values expressed as median (10-90% CI)FMVD: Flow-mediated vasodilation. BMI: body mass index
In the first logistic regression analysis, BAD was independently associated with MS, with OR 1.42 (95% CI 1.14-1.77, p=0.002), adjusted for age, gender, BMI, dyslipidemia, smoking, DM, HTN and sedentary lifestyle [Hosmer-Lemeshow test p=0.12, area under the ROC curve 0.77 (95% CI 0.75-0.79)] (Table 2).
Table 2 Adjusted brachial artery diameter and its relationship with metabolic syndrome (MS).
| Variable | Beta coefficient | Standard error | p | OR | 95% CI |
|---|---|---|---|---|---|
| Age | 0.009 | 0.006 | 0.125 | 1.01 | 0.99-1.02 |
| Male gender | 0.325 | 0.169 | 0.054 | 1.38 | 0.99-1.92 |
| BMI | 0.115 | 0.013 | <0.0001 | 1.12 | 1.09-1.15 |
| BAD | 0.350 | 0.112 | 0.002 | 1.42 | 1.14-1.77 |
| DLP | 0.384 | 0.134 | 0.004 | 1.47 | 1.12-1.91 |
| SMK | -0.119 | 0.179 | 0.503 | 0.88 | 0.62-1.26 |
| DM | 0.630 | 0.263 | 0.016 | 1.87 | 1.12-3.14 |
| HTN | 0.773 | 0.132 | <0.0001 | 2.16 | 1.67-2.80 |
| Sedentary lifestyle | 0.134 | 0.143 | 0.347 | 1.14 | 0.86-1.51 |
BMI: Body mass index; BAD: Baseline brachial artery diameter; DLP: Dyslipidemia; DM: Diabetes mellitus; HTN: Hypertension; SMK: Smoking
In the second logistic regression analysis, FMVD did not show a significant association with MS, with OR 0.98 (95% CI 0.97-1.007, p=0.217) when adjusted for the same variables [Hosmer- Lemeshow test p=0.15, area under the ROC curve= 0.77 (95% CI 0.75-0.79)] (Table 3).
Table 3 Relationship between adjusted flow-mediated vasodilation and metabolic syndrome (MS).
| Variable | Beta coefficient | Standard error | p | OR | 95% CI |
|---|---|---|---|---|---|
| Age | 0.012 | 0.005 | 0.024 | 1.01 | 1.01 - 1.02 |
| Male gender | 0.641 | 0.135 | <0.0001 | 1.89 | 1.45 - 2.47 |
| BMI | 0,122 | 0.013 | <0.0001 | 1.13 | 1.10 - 1.15 |
| FMVD | -0.011 | 0.009 | 0.217 | 0.98 | 0.96 - 1.00 |
| DLP | 0.345 | 0.134 | 0.010 | 1.41 | 1.08 - 1.83 |
| SMK | -0.122 | 0.180 | 0.497 | 0.88 | 0.62 - 1.26 |
| DBT | 0.662 | 0.261 | 0.011 | 1.93 | 1.16 - 3.24 |
| HTN | 0.772 | 0.132 | <0.0001 | 2.16 | 1.67 - 2.81 |
| Sedentary lifestyle | 0.103 | 0.143 | 0.472 | 1.10 | 0.83 - 1.46 |
BMI: Body mass index; DLP: Dyslipidemia; DM: Diabetes mellitus; FMVD: Flow-mediated vasodilation; HTN: Hypertension; SMK: Smoking
DISCUSSION
Eccentric vascular remodeling, characterized in this study as an increase in baseline BAD, was associated with the development of MS components with the progressive increase in body mass. Thus, the inverse relationship between vascular remodeling (BAD) and FMVD (mechanism that increases vascular flow in situations of high metabolic demand, such as exercise and the postprandial period) could be linking hemodynamic dysfunction with metabolic alterations in the context of weight gain. Although the impact of metabolic alterations on vascular structure and function is unobjectionable, it is highly probable that at some point in the evolutionary continuum, the alterations in vascular dynamics contribute, in a vicious circle, to the development/enhancement of those metabolic imbalances.
Thus, eccentric remodeling would represent an attempt to ensure adequate muscle perfusion in response to both the increase in intravascular volume and the oversupply of high-energy substrates. Actually, sugars such as glucose 8 or fructose 9 increase the activity of the sympathetic system, with increased cardiac output - a cause of eccentric vascular remodeling - and increased tone of the precapillary sphincters with muscle hypoflow. These adjustments are associated with a drop in the consumption of energy substrates - glucose and triglycerides - and finally with the development of peripheral metabolic alterations. Effectively, different drugs that share sympatholytic/ vasodilatory effects such as moxonidine 10, rilmenidine 11 and azelnidipine, 12 or nitric oxide donors such as sodium nitroprusside 13 induce improvements in the glycemic and lipid profile in subjects with obesity, MS, or DM.
The association between the observed increase in BAD and its relationship with metabolic alterations does not seem to be a generalized phenomenon with the increase in body mass. In fact, and as an example, 1 in 3 obese people exhibit a “metabolically healthy” phenotype in which no evident metabolic alterations are manifested. 14 These subjects apparently present as distinctive characteristics an adequate subcutaneous lipid reservoir capacity, normally functioning adipocytes, and low levels of inflammation, all in the context of adequate muscle perfusion and microvascular function.
This observation would be in line with recent studies suggesting that adipose tissue oxygenation, determined by the balance between oxygen supply and oxygen consumption, could be a key factor in determining the adipose tissue phenotype in obese subjects. 15,16
It is highly probable that alterations in vascular function could be linked to the development of modifications in the metabolism of both carbohydrates and lipids in metabolically complicated subjects. Recent studies show that vascular insulin resistance secondary to high-fat diets compromises even short-term skeletal vascular flow. These data suggest that the alteration of blood flow in the skeletal muscle microvasculature is a primary event that would precede whole body insulin resistance in the development of type II DM. 17
In the same sense, in a large prospective population- based study conducted in middle-aged subjects, the elevated baseline levels of 2 markers of endothelial dysfunction (sE-selectin and sICAM-1) were significantly associated with the risk of developing type II DM, especially sE-selectin, which was found to be a strong independent predictor of incident DM after adjusting for obesity, other clinical parameters and lifestyle in both men and women. 18
Concomitantly, evidence has emerged that seems to link fatty deposit hypoperfusion with the development of total body insulin resistance. The inverse association between postprandial increase in adipose tissue perfusion with the degree of insulin resistance suggests that adipose tissue hypoperfusion may affect whole-body insulin sensitivity. Thus, the drop in the supply of glucose, lipids and oxygen to the adipose tissue would generate a decrease in the uptake of energy substrates at the level of subcutaneous fatty deposits, with ectopic accumulation of lipids in viscera and muscle, adipocyte hypoxemia and central and peripheral insulin resistance. 19
From our point of view, we have observed an interesting association between alterations in the vascular structure and function of the conductance vessels and metabolic abnormalities with weight gain. However, these associations should be interpreted with caution due to the inherent limitations of the study. Actually, with a design which does not allow causality to be imputed, the absence of an objective measurement of fat mass, and the fact that FMVD was not measured during the postprandial period or adjusted for drugs limit the interpretation of the results. However, it is probable that dynamic alterations in vascular flow behave as inducers of metabolic disorders, particularly during the postprandial period. Recent evidence shows that a fundamental difference between metabolically healthy obese subjects and those with MS lies in a preservation of FMVD during the postprandial period in those metabolically uncomplicated, with an endothelial response similar to that of subjects with normal weight and without metabolic alterations. 20 These findings are reinterpreting the function of the vascular endothelium as a fundamental actor in the control of glucose and carbohydrate metabolism. 21 We must also point out that in the present study the adjustment of hemodynamic variables for each of the drugs was not performed, with the intention of not making the analysis more complex. The study was carried out without the morning administration of the patients' usual medication, which would at least limit the immediate effect of high plasma drug levels.
Finally, it is not possible to completely rule out the fact that the increase in arterial conductance is mainly linked to anatomical modifications secondary to the increase in body mass. Effectively, in a study by Dalli et al. 22 a significant relationship was evidenced between BAD and the arm perimeter. However, the persistence of the association between BAD and MS adjusted by BMI, a parameter closely related with body surface area and, therefore, with arm circumference, would suggest an association independent of anthropometry in this relationship.
CONCLUSIONS
In the present study we have evidenced a relationship between vascular structure and function alterations in conductance arteries with metabolic disorders through increased body mass. A prospective study with the purpose of evaluating insulin-vascular resistance and hemodynamic-metabolic causality with weight gain in subjects prior to the development of MS could help clarify these associations.
BIBLIOGRAFIA
1. DeFronzo R, Cooke C, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest 1975;55:845-55. https://doi.org/10.1172/JCI107996 [ Links ]
2. Hall E, do Carmo J, da Silva A, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116:991-1006. https://doi.org/10.1161/CIRCRESAHA. 116.305697 [ Links ]
3. Hall M, do Carmo J, da Silva A, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014;7:75-88. https://doi.org/10.2147/IJNRD.S39739 [ Links ]
4. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012;59:1069-78. https://doi.org/10.1161/HYPERTENSIONAHA.111.190223 [ Links ]
5. Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123-9. https://doi.org/10.1152/ajpendo.00021.2003 [ Links ]
6. DeFronzo R. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773-95. https://doi.org/10.2337/db09-9028 [ Links ]
7. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al; International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-5. https://doi.org/10.1161/CIRCULATIONAHA.109.192644 [ Links ]
8. Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reprod Nutr Dev. 1996;36:391-7. https://doi.org/10.1051/rnd:19960405 [ Links ]
9. Tran L, Yuen V, McNeill J. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 2009; 332:145-59. https://doi.org/10.1007/s11010-009-0184-4 [ Links ]
10. Topal E, Cikim AS, Cikim K, Temel I, Ozdemir R. The effect of moxonidine on endothelial dysfunction in metabolic syndrome. Am J Cardiovasc Drugs. 2006;6:343-8. https://doi.org/10.2165/00129784-200606050-00007 [ Links ]
11. De Luca N, Izzo R, Fontana D, Iovino G, Argenziano L, Vecchione C. Haemodynamic and metabolic effects of rilmenidine in hypertensive patients with metabolic syndrome X. A double-blind parallel study versus amlodipine. J Hypertens. 2000;18:1515-22. https://doi.org/10.1097/00004872-200018100-00021 [ Links ]
12. Shimada K, Miyauchi K, Daida H. Azelnidipine and glucose tolerance: possible indications and treatment selection for hypertensive patients with metabolic disorders. Expert Rev Cardiovasc Ther. 2015;13:23-31. https://doi.org/10.1586/14779072.2015.986464 [ Links ]
13. Henstridge DC, Kingwell BA, Formosa MF, Drew BG, McConell GK, Duffy SJ. Effects of the nitric oxide donor, sodium nitroprusside, on resting leg glucose uptake in patients with type 2 diabetes. Diabetologia 2005;48:2602-8. https://doi.org/10.1007/s00125-005-0018-1 [ Links ]
14. Van Vliet-Ostaptchouk J, Nuotio M, Slagter S, Doiron D, Fischer K, Foco L, et.al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord 2014;14:9. https://doi.org/10.1186/1472-6823-14-9 [ Links ]
15. Goossens G, Blaak E. Adipose tissue oxygen tension: implications for chronic metabolic and nflammatory diseases. Curr Opin Clin Nutr Metab Care 2012;15:539-46. https://doi.org/10.1097/MCO.0b013e328358fa87 [ Links ]
16. Goossens G, Blaak E. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne) 2015;6:55. https://doi.org/10.3389/fendo.2015.00055 [ Links ]
17. Carmichael L, Keske M, Betik A, et.al. Is vascular insulin resistance an early step in diet-induced whole-body insulin resistance? Nutr Diabetes. 2022;12:31. https://doi.org/10.1038/s41387-022-00209-z [ Links ]
18. Thorand B, Baumert J, Chambless L, Meisinger C, Kolb H, Döring A, et.al. MONICA/KORA Study Group. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middleaged men and women from the general population. Arterioscler Thromb Vasc Biol. 2006;26:398-405. https://doi.org/10.1161/01.ATV.0000198392.05307.aa [ Links ]
19. Emanuel AL, Meijer RI, Muskiet MH, van Raalte DH, Eringa EC, Serné EH. Role of Insulin-Stimulated Adipose Tissue Perfusion in the Development of Whole-Body Insulin Resistance. Arterioscler Thromb Vasc Biol. 2017;37:411-8. https://doi.org/10.1161/ATVBAHA.116.308670 [ Links ]
20. Keirns BH, Hart SM, Sciarrillo CM, Poindexter KL, Clarke SL, Emerson SR. Postprandial triglycerides, endothelial function, and inflammatory cytokines as potential candidates for early risk detection in normal-weight obesity. Obes Res Clin Pract. 2022;16:386-92. https://doi.org/10.1016/j.orcp.2022.08.008 [ Links ]
21. Pi X, Xie L, Patterson C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ Res. 2018;123:477-94. https://doi.org/10.1161/CIRCRESAHA.118.313237 [ Links ]
22. Dalli E, Segarra L, Ruvira J, Esteban E, Cabrera A, Lliso R, et.al. Dilatación de la arteria humeral mediada por flujo en varones sanos, con factores de riesgo e infarto agudo de miocardio importancia de la posición del manguito oclusor. Rev Esp Cardiol. 2002;55:928-35. https://doi.org/10.1016/S0300-8932(02)76731-6 [ Links ]
Received: November 06, 2023; Accepted: January 15, 2024











 text in
text in 



