Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista de la Facultad de Ciencias Agrarias. Universidad Nacional de Cuyo
Print version ISSN 1853-8665On-line version ISSN 1853-8665
Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo vol.51 no.2 Mendoza Dec. 2019
DOSSIER IBAM
Effect of irrigation and soil texture on grape phylloxera (Daktulosphaira vitifoliae Fitch) population and grapevine damage
Efecto del riego y textura de suelo sobre la población de la filoxera de la vid (Daktulosphaira vitifoliae Fitch) y su daño en plantas
Celeste Arancibia 1, 4 *; Emiliano Malovini 2; Cecilia B. Agüero 3; Liliana Martínez 4
1 Becaria Doctoral Universidad Nacional de Cuyo-Corporación Vitivinícola Argentina (COVIAR). Mendoza. Argentina. * carancibia@fca.uncu.edu.ar
2 Becario Postdoctoral Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)-Universidad Nacional de Cuyo. Mendoza. Argentina.
3 University of California Davis. Department of Viticulture and Enology. One Shields Avenue. Davis. California 95616. USA.
4 Universidad Nacional de Cuyo. Facultad de Ciencias Agrarias. Cátedra de Fisiología Vegetal. Instituto de Biología Agrícola (IBAM). Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Almirante Brown 500. Chacras de Coria. Mendoza. M5528AHB. Argentina.
Originales: Recepción: 22/07/2019 - Aceptación: 29/10/2019
ABSTRACT
It is believed that phylloxera grows better in clay soils and/or under drip irrigation than in sandy soils and/or flooding. To test these hypotheses, phylloxera damage and population growth were evaluated in potted V. vinifera cv. Malbec under two irrigation methods and soil textures in growth chambers at 16 h of photoperiod and 28°C + 3°C. In a first experiment, phylloxera damage and population were analyzed in infested (P) and uninfested (C) plants, drip (D) or flood (F) irrigated. A second experiment consisted in infested (P) and uninfested (C) plants in clay (CL) or sandy (S) soil. D x P reduced leaf number, while P x C increased photosynthesis rate. In the irrigation experiment, P reduced leaf area, shoot length and root dry weight and increased stomatal conductance. Irrigation methods did not influence variables related to root damage or phylloxera population. In the texture experiment CL x C showed a greater leaf area. P also reduced shoot length and root dry weight while CL had a higher number of leaves and less root dry weight. Despite CL developed more phylloxera root symptoms, texture did not affect the number of insects found on roots. Possibly, neither irrigation methods nor soil texture per se are limiting factors for phylloxera performance, but their influence on the vigor of the plants could affect the plant-insect interactions. This is one of the first reports about the influence of soil conditions on phylloxera in a controlled environment and provides a foundation for further studies.
Keywords: Phylloxera; Daktulosphaira vitifoliae; Irrigation; Soil texture; Vitis vinifera
RESUMEN
Durante años, la afirmación sobre que la filoxera crece mejor en suelos arcillosos y/o bajo riego por goteo, que en suelos arenosos y/o riego superficial, se ha considerado cierta. En este experimento se evaluó el daño y la población de filoxera en V. vinifera cv. Malbec, en macetas bajo dos métodos de riego y texturas del suelo, en cámaras de crecimiento con 16 h de luz a 28°C + 3°C. En un primer experimento, los tratamientos consistieron en plantas infestadas (P) y no infestadas (C), regadas por goteo (D) o riego superficial (F). Otro experimento consistió en plantas infestadas (P) y no infestadas (C) en suelo arcilloso (CL) o arenoso (S). D x P disminuyó el número de hojas, mientras que F x C mostró una mayor tasa de fotosíntesis. P redujo el área foliar, longitud del brote y peso seco de la raíz y aumentó la conductancia estomática. Los métodos de riego no influyeron en las variables relacionadas con el daño de la raíz o la población de filoxera. En el segundo experimento CL x C mostró una mayor área foliar. P también redujo la longitud del brote y el peso seco de la raíz mientras que CL mostró más hojas y menos peso seco de la raíz. A pesar de que CL desarrolló más síntomas de filoxera en la raíz, la textura no afectó la cantidad de insectos encontrados en las mismas. Posiblemente, ni el método de riego ni la textura del suelo per se sean factores limitantes para la filoxera, pero su influencia sobre el vigor de las plantas podría afectar las interacciones planta-insecto. Este es uno de los pocos estudios en macetas y condiciones controladas que estudia la influencia de los factores de campo sobre la filoxera proporcionando las bases para futuros estudios.
Palabras clave: Filoxera; Daktulosphaira vitifoliae; Riego; Textura de suelo; Vitis vinifera
INTRODUCTION
Grape phylloxera (Daktulosphaira vitifoliae Fitch; Hemiptera; Sternorrhyncha; Phylloxeridae) is an obligated biotrophic monophagus insect which feeds on Vitis spp. producing leaf galls (mainly in native American Vitis spp.) and root nodosities and tuberosities (mainly in non-American Vitis spp.). Native to North America, the pest coevolved with American Vitis spp. and the severe damage apparently only occurs under cultivated situations (17). Grape phylloxera (from now on: phylloxera), as other root herbivores, lives in constant physical contact with the surrounding soil; therefore variables such as climate, vegetation and cultural practices that affect the spatial-temporal dynamics of the 3 phases that make up the soil (solids, water and gas) (34), also influence phylloxera's behavior. Soils are a particular heterogeneous environment and the range of abiotic conditions that influence phylloxera could be both diverse and complex (4). Some of these factors could be manipulated to control phylloxera; however, information regarding these interactions is scarce and poorly understood.
Soil texture and structure affect root herbivore insects’ physiology by modeling other abiotic attributes (4). For instance, water retention of fine-textured soils (6) makes these soils less prone to desiccation, increasing survivorship of several insect species (4) while coarse-textured soils normally are associated to negative impacts on root herbivore physiology. One example of the latter is the survivorship decrease of Diabrotica undecimpunctata (Coleoptera) larvae in sandy soils, which could be caused by the abrasiveness of the sand particles on their cuticle (33). In the case of phylloxera, soil texture could affect survivorship and dispersion; however, this phenomenon has not been widely studied. Chitkowski and Fisher (2005) suggested that the information that has led to believe that some type of soils, especially sandy soils and soilless agricultural substrates, do not admit D. vitifoliae populations is anecdotic. Probably the most cited literature regarding this topic is based on researches by Nougaret and Lapham (1928) and Davidson and Nougaret (1921). In these articles authors speculated that sandy soils would constrain phylloxera movement through the soil due to the lack of cracks, resulting in the immunity of vines growing in sandy soils. Also, de Klerk (1974) found a relationship between high levels of phylloxera infestation in heavy-textured soils and low levels of infestation in lighter textured soils in South African vineyards. Furthermore, in Australian vineyards Powell et al. (2003) also found a relationship between soil type and levels of infestation, although they could not attribute the observed differences to texture. In contrast, Buchanan (1987) could not find this association in other Australian vineyards, and Chitkowski y Fisher (2005) found that the insect was able to develop in 6 different types of soils and a soilless mix. Similarly, a link between soil texture and phylloxera infestation levels has not been established in Argentinean vineyards (3).
Water is another crucial factor influencing soil dynamics and, in this context, winter flooding has been used as a first attempt to eradicate phylloxera due to its hypothetical drowning and dragging effect. However, laboratory essays have shown that phylloxera can survive up to 21 days submerged under water (29) and a 24 h stream of water, applied directly upon phylloxera has been ineffective to remove it from the roots (10). Inundation could cause an indirect benefit by promoting an increase in the vine's vigor, but it is only possible to use it in non-permeable soils, it incurs in high economical costs, and it requires access to great amounts of water and additional fertilization to replace the soluble nutrients lixiviated out of the soil profiles (28). Moreover, the efficacy of such practice could depend on the phylloxera strain virulence and other biotic and abiotic factors.
In Argentina, phylloxera is present in most viticultural regions (13). For years, it has been commonly stated that since local vineyards are mainly flood irrigated and soils are mostly sandy or loam-sandy, phylloxera do not affect the vines to the point of big economical losses, even though about 90% of vineyards are own-rooted V. vinifera (23). Again, we have not found associations between phylloxera infestation levels and irrigation methods (drip or flood) in the Argentinean viticultural areas (3). Interestingly, most of the infested vineyards did not present visual aerial symptoms and the pest was unnoticed (Arancibia, 2013- 2019 personal observations). However, current changes in viticultural practices, especially the conversion from flood irrigation to drip irrigation could impact phylloxera behavior and change this scenario (2). In view of this background, climate change and its potential consequences in water availability, especially in irrigated areas (20), will force the preference of more efficient watering systems such as drip, therefore it is essential to have scientific information, about the interaction between soil pests and irrigation, and other abiotic factors such as soil textures.
In this work, we examined the effect of irrigation (drip and flood) and soil texture (clay and sandy) on phylloxera population growth and vine damage, in two different experiments, under controlled conditions, using V. vinifera cv. Malbec, the emblematic grape of Argentina. A better understanding of these interactions is essential for decision-making regarding water, soil and pest management at vineyard planting and in established vineyards and it will have long term implications for the economy of the industry.
MATERIALS AND METHODS
Plant material
Wood cuttings of V. vinifera cv. Malbec (clon 12 INTA) from the experimental field of Cátedra de Fisiología Vegetal-Facultad de Ciencias Agrarias (Mendoza, Argentina) were forced to root by submerging the base in 1000 ppm IBA (indol-butiric acid) 40% hydro-alcoholic solution for 10 seconds followed by culture in perlite, in a rooting chamber. Cutting bases were kept at 20 ± 2°C to stimulate callus formation and rootlet emission, while the upper parts were kept at 10 ± 2°C to prevent bud break. The photoperiod regime was 16 h light and 8 h of darkness. When roots were approximately 4 cm long, vines were transferred to 4.4 L pots with corresponding soil texture. Plants were grown in a growth chamber with 16 h light and 8 h of darkness and an average temperature of 28°C ± 3°C and light intensity of 40 μEinstein m-2 s-1. Vines were trained to a single shoot and selected for inoculation 3 months after transplant.
Inoculation
Phylloxera eggs were obtained from Argentinean genotype B grape phylloxera (3) grown on Malbec excised roots in Petri dishes at 24°C± 2°C, in the darkness. Fifty phylloxera eggs were transferred to a 1.5 μL Eppendorf® tube, which was subsequently laid down approximately 4 cm under the soil surface, in the central part of the pot, close to the roots. Inoculation was repeated 3 weeks later to insure effective infestation (9). In order to prevent insects from escaping and/or infesting control plants, all pots were wrapped and closed to the base of the plant with frost cloth. Plants were pruned at 30 cm together with the first inoculation.
Irrigation experiment
Plants were potted in sterile loam soil. Twenty-four vines were infested with phylloxera eggs and other 24 plants were left non-infested as control plants. Twelve infested and 12 uninfested plants were drip irrigated, while other 12 infested and 12 uninfested plants were surface watered. Irrigation treatments started together with first inoculation. Drip irrigation method consisted of 350 mL of water applied by plastic drip lines, with drippers (2.1 L h-1). Flood irrigation consisted of 350 mL of water applied by a dripping plastic hose, with bubblers (10.5 L h-1). In this case, the water flow was higher than soil basic infiltration, so the volume between the pot rim and the soil surface was full of water during irrigation; in drip irrigated pots, due to lower water flow of the drippers, water penetrated the soil immediately. Plants were watered twice per week. Differences in humidity profiles between irrigation methods were measured with Decagon DS5 (Decagon devices Inc., Washington, USA) humidity sensors (figure 1) showing a differential water distribution in the pots of the different irrigation methods.
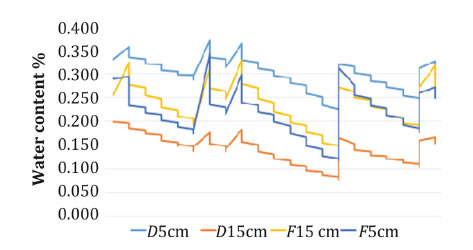
Figure 1. Volumetric water percentage measured every 24 hours in pots under drip (D) and flood (F) irrigation.
Figura 1. Porcentaje de volumen de agua medido cada 24 horas en macetas bajo riego por goteo (D) y riego superficial (F).
Six pots were placed under 8 irrigation lines each, alternating drip and flood methods. In each irrigation line infested and control plants were placed randomly. Treatments were designated infestation (phylloxera (P) and control (C)) and irrigation method (flood (F) and drip (D)). Analysis included only data from plants in which all variables were measured. For this reason, the final number of replicates was: 7 F x P, 5 F x C, 6 D x P and 7 D x C.
Soil texture experiment
Plants were potted in sterile agricultural sandy or clay soil (86 mL and 102 mL sediment volume, respectively) three months before inoculation. Fourteen plants in clay and 16 in sandy soil were infested with phylloxera and 14 plants in clay and 16 in sandy soil were left uninfested as control plants. Plants in sandy soil were watered with 200 mL and plants in clay soil with 350 mL to reach field capacity, about twice per week depending on the atmospheric demand.
Pots were randomly arranged in the growth chamber. Treatments were designated infestation (phylloxera (P) and control (C)) and soil texture (sandy (S) and clay (CL)). Analysis included only data from plants in which all variables were measured. For this reason, the final number of replicates was: 6 CL x P, 8 CL x C, 6 S x P and 5 S x C.
Measured variables
In both experiments, main shoot length, number of leaves and relative chlorophyll content (RCC) were measured 60 and 90 days after inoculation (dai). RCC was measured in leaves from position 4 to 8, counting from the apex, using a SPAD-502 Chlorophyll Meter (Minolta Corp., Ramsey, NJ) and data was averaged to yield a single value per vine. Stomatal conductance was measured at the same time than RCC, using a Leaf Porometer SC-1 (Decagon devices Inc., Washington, USA), in leaves from position 3 to 5, counting from the apex, and then averaged to yield a single value per vine. Photosynthetic rate was assessed using a CIRAS-2 Portable Photosynthesis System (PP Systems, Amesbury, USA), 120 dai. Plants were removed from the pots 120 dai to count nodosities, tuberosities, phylloxera eggs, nymphs, and adults on the roots under a Carl Zeiss stereo microscope 35X. Total individuals were calculated as the sum of eggs, nymphs and adults. Root dry weight (RDW) was assessed by placing roots at 55°C until constant weight. Number of eggs, nymphs, adults and total individuals were related to RDW. Total leaf area was measured with a LI-COR 3000ª (LI-COR, Inc., Lincoln, USA) and then dried at 55°C until constant weight to calculate leaf dry weight (LDW) and LDW/total leaf area ratio. A similar procedure was followed to determine shoot dry weight (SDW).
Statistical analysis
Principal coordinate analyses (PCoA) were performed to examine variables in reduced dimensions in both experiments. Photosynthetic rate (mmol CO2 m-2 s-1), number of leaves, stomatal conductance (mmol H2O m-2 s-1), leaf area (cm2), LDW/total leaf area ratio (g/cm2), RCC (SPAD unities), SDW (g), main shoot length (cm) and RDW (g) were included in the PCoA to evaluate the effects of phylloxera on the aerial part of the plants. In this analysis, for those variables measured more than once, only last measurement was included. Data was standardized, Euclidean distance was used and the distance function applied was Mij=-0.5*Dij*Dij. Similarly, PCoA was applied to number of eggs, nymphs, adults, total individuals, tuberosities and nodosities to analyze phylloxera population density and damage in roots. Data were analyzed by generalized linear mixed models (GLMM) and Akaike and Bayesian Information Criteria were applied to select the best models. Factors infestation, irrigation method and soil texture were computed as fixed effects. In the irrigation experiment, drip line (1 to 6) and pot locations in the growth chamber (border or interior) were computed as random effects. When variables were measured more than once, time was considered a fixed effect. An independent error correlation structure was applied and, when necessary, heteroscedasticity was corrected using the VarIdent function. Mean comparison was estimated by DGC test (14). All described statistical analyses were carried out using InfoStat v. 2017 software (15). In the soil texture experiment, inoculation of roots was not 100% successful and Fisher's exact test (1) was applied to examine the statistical association between soil texture and infestation success using www.langsrud.com/fisher.htm.
RESULTS AND DISCUSSION
For many years, it has been widely accepted that phylloxera prefers heavy clay soils and that flood irrigation can help to lower the population levels. Although these claims are supported by several reports (10, 12, 25, 31), many others have refuted them (8, 9, 27); so, more studies are needed to clarify these interactions. In this project we present the results obtained from two experiments involving the interaction between irrigation and soil texture with phylloxera population and grapevine damage.
Table 1 (page 443) shows, GLMM results of the statically significant variables found in the irrigation experiment; results of non-significant analyses (p > 0.05) are not shown.
Table 1. Means, P values and standard errors for irrigation experiment significant variables.
Tabla 1. Medias, valores P y errores estándar para las variables significativas del experimento de riego.
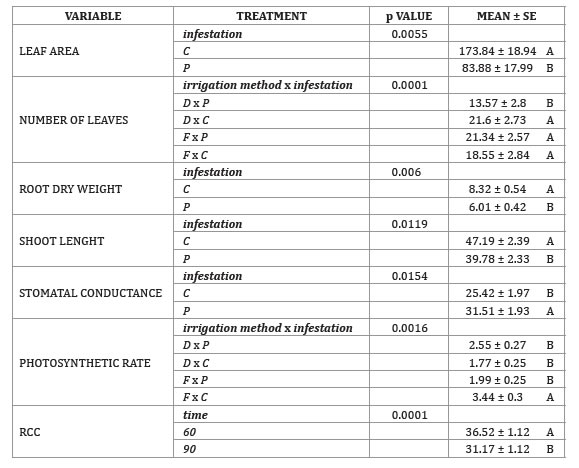
SE: standard error. Same letters do not represent a statistical difference. DGC test (p < 0.05).
MEAN: media; SE: error estándar. Letras iguales no presentan diferencias significativas. DGC test (p < 0,05)
As expected, total leaf area, RDW and shoot length were affected by infestation, showing that phylloxera damage was reflected in the aerial part of the plants.
Several significant interactions were also found. A reduction in number of leaves of D x P was possibly caused by water stress in parts of the root system, given the humidity gradient in the drip irrigated pots which, in addition to the interaction with the insect, could have affected leaf production or abscission.
The increase in photosynthetic rate in F x C, could be due to homogeneous water availability in the pots and to the fact that, even in small populations, phloem sucker insects can decrease the photosynthesis rate of their hosts (22) by repressing genes required by Rubisco and photosystem II activity (19). Furthermore, the healthy root system of control plants may have permitted an adequate water and nutrient intake that stimulated photosynthesis process. Also, in the field, leaf chlorosis is often the initial visual indication of phylloxera infestation; however, in this experiment phylloxera did not reduce RCC, in contrast to other studies (5). Only time reduced RCC as result of a natural ontogenic process. Phylloxerated plants showed reduction of 52% in leaf area. Similar results were obtained by with Eitle et al. (2017) in grafted and ungrafted rootstocks, however LDW/ total leaf area ratio remained unaffected. Phylloxera was responsible for 18% shoot length reduction and since the number of leaves remained unaltered, internodes were shortened (a common symptom in phylloxerated vineyards). A suboptimal cell elongation originated by a restriction of water intake due to a limited root system could cause this phenomenon. SDW was not affected by any treatment. Phylloxerated plants showed 28% less root mass than C plants, contrary to the increase found by Eitle et al. (2017), and our results can be attributed to a secondary pathogen attack (non-tested), a deviation of photoassimilates to biosynthetic pathways implicated in the plant’s defense mechanism or to a different grapevine genetic background used in this experiment. In general, the reduction of vines biomass in P can be explained by the metabolic cost incurred due to infestation (35); either as an infestation induced response (oxidative enzymes activity, hormones and phenolic compounds accumulation) (28), or as part of a phylloxera manipulation towards plant's metabolism genes to assure a source of nutrients for it (18). The increase of stomatal conductivity in P could be due to a larger stomatal cell opening or a higher density of stomata (not measured). As Nabity et al. (2013) observed, leaf phylloxera can manipulate grapevine primary metabolism and induce stomata formation on adaxial leaf surface, therefore it is possible that chemical signals related to phylloxera infestation move from the roots through the vascular system and induce stomata formation or regulate its opening. The multidimensional scaling approach by the PCoA revealed the grouping of D x P plants on the negative side of axis 1, while the rest of the combined treatments were almost exclusively on the positive side of the axis at random positions (figure 2).
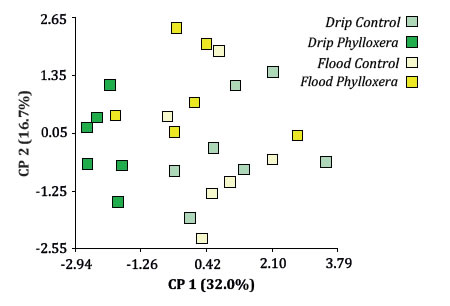
Figure 2. ACoP Biplot of photosynthetic rate; chlorophyll relative content; stomatal conductance; main shoot length; number of leaves; leaf area; main shoot length dry weight; leaf dry weight/leaf area ratio and root dry weight, using combined treatments as classification criterion.
Figura 2. Biplot del ACoP para las variables: tasa fotosintética, contenido relativo de clorofila, conductancia estomática, longitud de brote, número de hojas, área foliar, peso seco de brote, peso seco de raíces y área foliar/peso seco, tomando los tratamientos combinados como criterio de clasificación.
In general, the combined treatments were not able to predict the response of these variables (in concordance with the observed through GLMM in the individual analyses of the variables); the grouping of D x P plants must have responded to the stronger influence of number of leaves variable.
The number of insects at different life stages (eggs, nymphs, adults and total individuals) and the number of tuberosities and nodosities were not affected by the irrigation method and these results agree with previous observations in Argentinean vineyards, that did not find a statistical association between levels of infestation and type of irrigation (3). The biplot obtained from the multidimensional scaling approach of these variables (figure 3, page 445) when irrigation method was used as a classification criterion, did not show any obvious groupings, reveling again the lack of ability of F or D to predict the behavior of such variables under our experimental conditions.
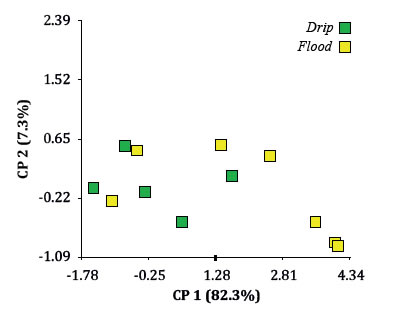
Figure 3. ACoP Biplot for variables measured on phylloxerated plants: tuberosities and nodosities, adults, nymphs, eggs and total individuals expressed per root dry weight, using irrigation method as classification criterion.
Figura 3. Biplot del ACoP para las variables de las plantas del tratamiento filoxera: tuberosidades y nudosidades, adultos, ninfas, huevos, individuos totales, todos expresados por peso seco de raíz en gramos tomando el factor método de riego como criterio de clasificación.
Many studies have revealed that the strength of plant-herbivorous relationship may be based on plant health (9). The Plant Vigor Hypothesis proposes that plants or plant organs that grow vigorously and are relatively large are favorable to herbivorous attack; in such interaction it is suggested that galling insects normally attack the most vigorous plants or plant organs (30). On the other hand, The Plant Stress Hypothesis proposes that plants undergoing some type of physiological stress are more susceptible to herbivory, given a reduction on protein synthesis and an increment of amino acids in tissues, thus generating an improved nutritious source of food for nitrogenlimited organisms (30).
In this study, although only photosynthetic rate mean was significantly higher in F x C, 6 other variables were also higher (non-significant) in F, including dry weights. These results suggest that The Plant Vigor Hypothesis could explain the tendency of F to harbor more insects, as found by Kimberling et al. (1990) in phylloxera leaf galling attacks.
To date, the predominant irrigation system in Argentina has been flooding. Water availability is subjected to climate and government regulations, among other factors. Nevertheless, even in regions where water availability, soil conditions and economic resources allow flooding, the killing of phylloxera under these circumstances is debatable. Most likely soil moisture is important at both ends of the spectrum, especially because of its influence on plant growth. That is, the direct effect of flooding on the insect would not be as important as the effect on vine physiology, whether the plant-insect interaction responds to the hypothesis of vigor or the hypothesis of the stress. Also, both irrigation methods present differences in cultural practices associated to soil management that can also affect phylloxera indirectly through variations in soil micro-flora and fauna.
GLMM results of the statically significant variables (p < 0.05) in the soil texture experiment are shown in table 2 (page 446) (non-significant analyses are not shown).
Table 2. Mean, P value and standard error for soil texture experiment significant variables.
Tabla 2. Medias, valores P y errores estándar para las variables significativas del experimento de texturas de suelo.
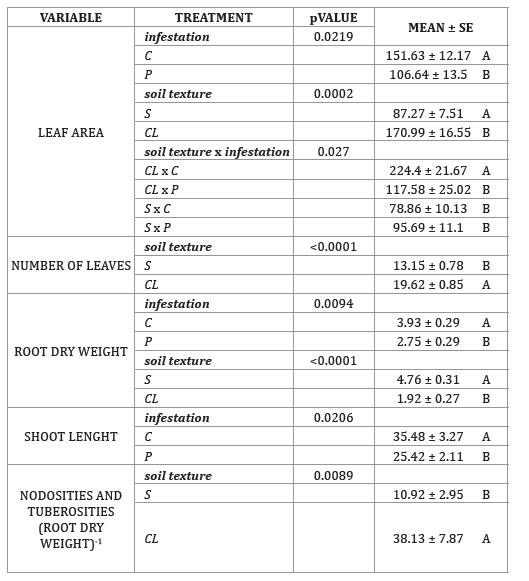
SE: standard error. Same letters do not represent a statistical difference. DGC test (p < 0.05).
MEAN: media; SE: error estándar. Letras iguales no presentan diferencias significativas. DGC test (p < 0,05)
Clay Control plants showed a larger leaf area than the rest of the combined treatments. This could be the effect of the greater number of leaves observed in CL plus a healthy root system growing in a soil with higher water retention capacity than a sandy soil (32). The steady water supply would provide the necessary turgor for cell enlargement and maximum leaf expansion. Grapevines under CL generated around 33% more leaves than those in S. It is possible that the lower water retention and field capacity in S could have caused temporal water stress events, and consequently, induced abscisic acid synthesis followed by stomata closure and a decrease of CO 2 uptake. Decreased photosynthesis would have, in turn, inhibited leaf production, as a strategy to prevent water loss through the canopy (11).
As observed in the irrigation experiment, P also decreased root dry weight by 30%. Similar results were reported by Omer et al. (1995) in a pot trial with V. vinifera cv. Chardonnay. Furthermore, this variable was 40% lower in CL, supporting the prediction of smaller root systems in fine-textured soils (32). It is possible that the higher water content available for plants growing in CL favored carbohydrate partitioning to the shoot over the root.
As in the irrigation experiment, shoot length was also reduced around 28% by phylloxera. The analysis of all these variables through a multifactorial approach, showed a biplot where CL x P and CL x C appeared mainly on the positive side of principal coordinate 1 and S x P and S x C on the negative side (figure 4, page 446) , which suggests that only texture was able to predict the response of such variables.
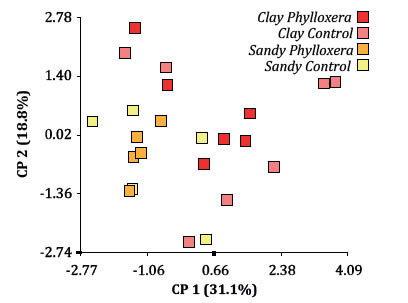
Figure 4. ACoP Biplot of photosynthetic rate; chlorophyll relative content; stomatal conductance; main shoot length; number of leaves; leaf area; main hoot length dry weight; leaf dry weight/leaf area ratio and root dry weight, using combined treatments as classification criterion.
Figura 4. Biplot del ACoP para las variables: tasa fotosintética, contenido relativo de clorofila, conductancia estomática, longitud de brote, número de hojas, área foliar, peso seco de brote, peso seco de raíces y área foliar/peso seco, usando los tratamientos combinados como criterio de clasificación.
This was supported by the fact that soil texture statically influenced 6 variables, while infestation affected 3, and soil texture x infestation only one.
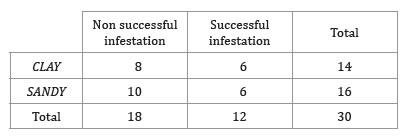
In this trial, soil texture did not affect egg survival, hatching and survival of the nymphs, as revealed by Fisher’s exact test; there was no association between soil texture and phylloxera infestation success (p = 1) (table 3).
Table 3. Number of successful and non-successful infested plants in clay and sandy soil.
Tabla 3. Número plantas con infestaciones exitosas y no exitosas en macetas de suelo arcilloso y arenoso.
Phylloxera life stage means did not differ between soil texture treatments (p > 0.05). These results are supported by Chitkowski and Fisher (2005) who testing 8 different types of soils from Oregon and Washington found a normal phylloxera growth population in all of them. Unexpectedly, CL plants had more nodosities and tuberosities than those in S, however this response did not appear to affect plant growth. Again, even under infestation it is possible that better water availability had promoted vine growth and improved tolerance to the pest.
The fact that more root symptoms in CL were not accompanied by a larger phylloxera population could be explained by the proposed physical resistance of sandy soil to phylloxera movement. It is possible that in CL, phylloxera could have moved easier to fresh roots when root deterioration progressed. On the contrary, S would have limited such mobility. Finally, the biplot did not expose clear groupings between levels, when variables related to phylloxera population and root damage were analyzed by PCoA, using soil texture as classification criterion (figure 5).
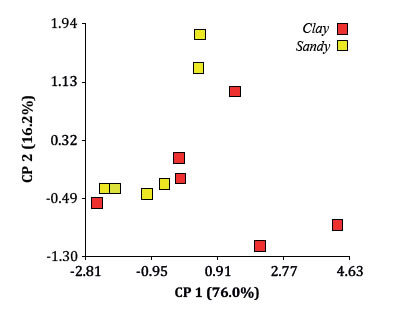
Figure 5. ACoP Biplot for variables measured on phylloxerated plants: tuberosities and nodosities, adults, nymphs, eggs and total individuals expressed per root dry weight, using soil texture as classification criterion.
Figura 5. Biplot del ACoP, Análisis para las variables de las plantas del tratamiento filoxera: tuberosidades y nudosidades, adultos, ninfas, huevos, individuos totales, todos expresados por peso seco de raíz en gramos tomando el tratamiento textura de suelo como criterio de clasificación.
The results of this experiment contradict the findings of Nougaret and Lapham (1928), who concluded that sandy soils prevent or suppress phylloxera establishment (9). Other reports have also suggested that sandy soil do not inhibit phylloxera growth (3, 8, 9, 27).
The results of this study suggest that the irrigation method and soil texture have little incidence on phylloxera development, at least under these experimental conditions. This is in agreement with field observations in the Argentinean viticultural area, which revealed that phylloxera exists in a broad type of texture and compaction degree spectrum and did not find associations between infestation level and soil texture or irrigation method (3).
Nougaret and Laphman (1928) found that in deep, friable and recent-alluvial soils with porous subsoils, destruction caused by phylloxera infestations is slow. This could be the case of Argentinean vineyards, mostly located in Entisols y Aridisols (of different suborders), generally unstructured, with depths up to 2 meters, which allow good infiltration and an optimal development of the root system.
Non-specific stress symptoms are difficult to separate from other conditions ranging from water or nutrient stress to phylloxera or disease burden (7). Moreover, the dynamics of vineyard soils can be as diverse as the factors influencing them and their numerous combinations. This complexity makes hard to establish a direct relation between a specific factor and its influence on phylloxera's population. It is possible that texture and irrigation of the soil might play an important role in the dispersion, rather than in phylloxera survival. Possibly, these influences are indirect, and interact with other factors involved in the plantsoil-insect system rather than as a per se factor. For instance, is likely that other ecological actors such as soil bacteria, mycorrhiza and other soil fungus play an important role as beneficial or harmful agents under different soil textures and humidity conditions, affecting vine and phylloxera physiology. Under The Plant Vigor Hypothesis, synergy between factors such as low incidence of biotic diseases, high energy supply, optimum climate, good cultural management, deep soils and adequate irrigation, create a perfect combination for the development of a good plant defense system able to cope with the plague's attack and may be the key for the coexisting situation of phylloxera and Vitis spp., at levels that do not generate big economical losses. In view of these results and the accumulated experimentation of other researchers, field experimentation based on an adequate and precise methodology is essential to formulate accurate conclusions of these phenomena. Since water resources will be challenged by the effects of climate change (20), its management will be a critical factor in the years to come, at regional and global scale. Understanding the plant-soil-water-insect interactions under field conditions is crucial for an effective and efficient water management and the sustainability of the grape industry.
CONCLUSIONS
The effects of two irrigation methods and soil textures were studied on V. vinifera cv. Malbec in pots under controlled conditions. Phylloxerated plants under drip irrigation decreased the number of leaves while uninfested plants under flood had a higher photosynthetic rate. Phylloxera itself reduced leaf area, shoot length and root dry weight and increased stomatal conductance. Irrigation methods did not affect variables related to root damage or phylloxera population. On the other hand, uninfested plants on clay soil had a greater leaf area and more leaves. Phylloxera also reduced shoot length, root dry weight while plants on clay soil showed less root dry weight. Finally, more phylloxera root symptoms were found in plants in clay soil; however, texture did not affect the number of insects. Moreover, after inoculation soil texture had no influence on the infestation success of the plants.
It is possible that neither irrigation methods nor soil texture per se are a limiting factor for phylloxera performance, however their influence on the vigor of the plants will affect plant interactions with the pest. It is possible that the growing conditions present in Argentinean vineyards promote a balance in which the health of the vineyards is not dangerously compromised. This is the first study that analyzes the effects of irrigation and soil texture using Argentinian phylloxera strains and can constitute the base for field trials necessary to bring integrative results to develop phylloxera control management strategies in Argentina.
1. Agresti, A. 1992. A survey of exact inference for contegency tables. Statitical Science. 7: 131-153. [ Links ]
2. Agüero, C. B.; Alonso, R.; Buscema, F.; Martínez, L.; Arancibia, C.; Lund, K.; Riaz, S.; Walker, M. A. 2017. Molecular characterisation of phylloxera present in Argentinean vineyards. Acta Horticulturae. 1157: 273-276. [ Links ]
3. Arancibia, C.; Riaz, S.; Agüero, C.; Ramírez-Corona, B.; Alonso, R.; Buscema, F.; Martínez, L.; Walker, M. A. 2018. Grape phylloxera (Daktulosphaira vitifoliae Fitch) in Argentina: ecological associations to diversity, population structure and reproductive mode. Australian Journal of Grape and Wine Research. 24(3): 284-291. [ Links ]
4. Barnett, K.; Johnson, S. N. 2013. Living in the soil matrix: Abiotic factors affecting root. In: Johnson, S. N.; Hiltpold, I.; Turlings, T. C. J. (Eds.). Advances in insect physiology. Behaviour and Physiology of Root Herbivores. Oxford. England. Academic Press. Elsevier. 159-218. [ Links ]
5. Blanchfield, A. L.; Robinson, S. A.; Renzullo, L. J.; Powell, K. S. 2006. Phylloxera-infested grapevines have reduced chlorophyll and increased photoprotective pigment content - can leaf pigment composition aid pest detection? Functional Plant Biology. 33: 507-514. [ Links ]
6. Brady, N. C.; Weil, R. R. 2007. The nature and properties of soils. 14th edition. New Jersey. USA. Pearson Education Prentice Hall. [ Links ]
7. Bruce, R. J.; Powell, K. S.; Norng, S.; Robinson, S. A. 2011. Grapevine leaf pigment response to root infestation by phylloxera. Acta Horticulturae. 904: 93-99. [ Links ]
8. Buchanan, G. A. 1987. The distribution of grape phylloxera, Daktulosphaira vitifolii (Fitch), in central and north-eastern Victoria. Australian Journal of Experimental Agriculture. 27: 591-595. [ Links ]
9. Chitkowski, R. L.; Fisher, J. R. 2005. Effect of soil type on the establishment of grape phylloxera colonies in the Pacific Northwest. American Journal of Enology and Viticulture. 56: 207-211. [ Links ]
10. Davidson, W. M.; Nougaret, R. L. 1921. The grape phylloxera in California. USDA Bulletin. 903 p. [ Links ]
11. Davies, W. J.; Wilkinson, S.; Loveys, B. 2002. Stomatal control by chemical signalling and the exploitation of this mechanism to increase water use efficiency in agriculture. New Phytologist. 153: 449-460. [ Links ]
12. de Klerk, C. A. 1974. Biology of Phylloxera vitifoliae (Homoptera: Phylloxeridae) in South Africa. Phytophylactica. 6: 109-118. [ Links ]
13. del Toro, M. S.; García Sáez, J. G.; Larriqueta, J. E.; Castellanos, S. J.; Martinotti, M. D.; Marín, M. S.; Quercetti, M. J.; Lillo, M. I.; Moyano, E. A. M. 2007. Manejo de filoxera de la vid Daktulosphaira vitifoliae FITCH. In: II Encuentro Italo Argentino sobre la producción integrada de los cultivos: vides y vinos. Córdoba-Mendoza. Argentina. Edited by Cavallo, A.; Pasqualini, E; Passera, C. 159-176. [ Links ]
14. Di Rienzo, J. A.; Guzman, A. W.; Casanoves, F. 2002. A multiple comparisons method based on the distribution of the root node distance of a binary tree obtained by average linkage of the matrix of Euclidean distances between treatment means. Journal of Agricultural, Biological and Environmental Statistics. 7: 129-142. [ Links ]
15. Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; Gonzalez, L.; Tablada, M.; Robledo, C. W. InfoStat versión 2014. Grupo InfoStat. FCA. Universidad Nacional de Córdoba. Argentina. URL Available in: http://www.infostat.com.ar [ Links ]
16. Eitle, M. W.; Cargnoni, M.; Acar, A.; Martinez, S. C.; Failla, O.; Griesser, M.; Forneck, A. 2017. Phylloxeration effects on the sink activity and assimilation rate in phylloxera (Daktulosphaira vitifoliae Fitch) infested grapevines (Vitis spp.) Acta Horticulturae. 1188: 291-298. [ Links ]
17. Granett, J.; Walker, M. A.; Kocsis, L.; Omer, A. 2001. Biology and management of grape phylloxera. Annual Review of Entomology. 46: 387-412. [ Links ]
18. Griesser, M.; Caroline, N.; Crespo-Martinez, S.; Schoedl-hummel, K.; Wieczorek, K.; Gorecka, M.; Liebner, F.; Zweckmair, T.; Pavese, N. S.; Kreil, D.; Forneck, A. 2015. Phylloxera (Daktulosphaira vitifoliae Fitch) alters the carbohydrate metabolism in root galls to allowing the compatible interaction with grapevine (Vitis ssp) roots. Plant Science. 234: 38-49. [ Links ]
19. Heidle, A. J.; Baldwin, I. T. 2004. Microarray analysis of salicylic acid- and jasmonic acid- signalling in responses of Nicotiana attenuata to attack by insects from multiple feeding guilds. Cell and Environment. 27: 1362-1373. [ Links ]
20. IPCC. 2014: Cambio climático 2014. Impactos, adaptación y vulnerabilidad. Resúmenes, preguntas frecuentes y recuadros multicapítulos. Contribución del Grupo de trabajo II al Quinto Informe de Evaluación del Grupo Intergubernamental de Expertos sobre el Cambio Climático [Field, C. B., V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, M. Chatterjee, K. L. Ebi, Y. O. Estrada, R. C. Genova, B. Girma, E. S. Kissel, A. N. Levy, S. MacCracken, P. R. Mastrandrea y L. L. White (eds.)]. Organización Meteorológica Mundial, Ginebra (Suiza). 200 p. [ Links ]
21. Kimberling, D. N.; Scott, E. R.; Price, P. W. 1990. Testing a new hypothesis: plant vigor and phylloxera distribution on wild grape in Arizona. Oecologia. 84: 1-8. [ Links ]
22. Macedo, T. B.; Bastos, C. S.; Higley, L. G.; Ostlie, K. R.; Madhavan, S. 2003. Photosynthetic responses of soybean to soybean aphid (Homoptera: Aphididae) injury. Journal of Economic Entomology. 96: 188-193. [ Links ]
23. Mendoza, G.; Becerra, V.; Dagatti, C.; Herrera, M. E. 2013. La filoxera en Mendoza: actualización de una plaga olvidada. Ruralis. 17: 15-16. [ Links ]
24. Nabity, P. D.; Haus, M. J.; Berenbaum, M. R.; De Lucia, E. H. 2013. Leaf-galling phylloxera on grapes reprograms host metabolism and morphology. Proceedings of the National Academy of Sciences of the United States of America. 110(41): 16663-16668. [ Links ]
25. Nougaret, R. L.; Lapham, M. H. 1928. A study of phylloxera infestation in California as related to types of soils. US Department of Agricultural. Technical Bulletin. 20: 1-39. [ Links ]
26. Omer, A. D.; Granett, J.; De Benedictis, J. A.; Walker, M. A. 1995. Effects of fungal root infections on the vigor of grapevines infested by root-feeding grape phylloxera. Vitis. 34(3): 165-170. [ Links ]
27. Powell, K. S.; Slattery, W. J.; Deretic, J.; Herbert, K.; Hetherington, S. 2003. Influence of soil type and climate on the population dynamics of grapevine phylloxera in Australia. Acta Horticulturae. 617: 33-41. [ Links ]
28. Powell, K. S.; Cooper, P. D.; Forneck, A. 2013. The Biology, Physiology and Host–Plant Interactions of Grape Phylloxera Daktulosphaira vitifoliae. In: Johnson, S. N.; Hiltpold, I.; Turlings, T. C. J. (Eds.) Advances in insect physiology. Behaviour and Physiology of Root Herbivores. Oxford, England, Academic Press. Elsevier. 160-204.
29. Powell, K. S.; Bruce, R. J.; Korosi, G. A. 2014. Assessing the risk of phylloxera survival during white grape processing. Acta Horticulturae. 1045: 99-107. [ Links ]
30. Price, P. W. 1991. The plant and herbivore attack hypothesis. Oikos. 62(2): 244-251. [ Links ]
31. Rombough, L. 2002. The grape grower: A guide to organic viticulture. White River Junction, Vermont. Canada. Chelsea Green Publishing. [ Links ]
32. Smart, D. R.; Schwass, E.; Lakso, A.; Morano, L. 2006. Grapevine rooting patterns: A comprehensive analysis and a review. American Journal of Enology and Viticulture. 57(1): 89-104. [ Links ]
33. Turpin, F. T.; Peters, D. C. 1971. Survival of southern and western corn rootworm larvae in relation to soil texture. Journal of Economic Entomology. 64: 1448-1451. [ Links ]
34. Villani, M. G.; Wright, R. J. 1990. Environmental influences on soil macroarthropod behavior in agricultural systems. Annual Review of Entomology. 35: 249-269. [ Links ]
35. War, A. R.; Paulraj, M. G.; Ahmad, T.; Buhroo, A. A.; Hussain, B.; Ignacimuthu, S.; Sharma, H. C. 2012. Mechanisms of plant defense against insect herbivores. Plant Signaling and Behavior. 7(10): 1306-1320. [ Links ]
ACKNOWLEDGEMENTS
This paper is part of Celeste Arancibia doctoral CONICET fellow. We would like to thank undergraduate students from Facultad de Ciencias Agrarias-UNCuyo: Kevin Villalba, Paula Fianacca, Federico Carabajal, Fernanda Mauro, Ariel Cicchitti, Valentina Di Fabrizio and Guillermo Corti for their participation in the experiments.
This work was funded by the National University of Cuyo (Research Program SeCTyP - UNCuyo 06/A595 2013-2016), Mendoza, Argentina.














