Introduction
Reports of fish mortality are relatively frequent each year, occurring in both lotic and lentic ecosystems (González Naya et al. 2011, Gómez 2014, Agostinho et al. 2021). These occur for the action of certain environmental (Freyre 1967, Gómez 1986, Colautti et al. 1998, Mancini et al. 2000), biological (Grosman and Sanzano 2002) and anthropic factors (Agostinho et al. 2021). Furthermore, evidence supports the confluence of multiple factors contributing to fish mortality events (Langdon et al. 1985, Mancini et al. 2000, Hurst 2007, Noga 2010, Falkenberg et al. 2019). Environmental perturbations play a fundamental role in the appearance of diseases (Lafferty and Kuris 1999, Dar et al. 2019). Indeed, various opportunistic organisms thrives amidst environmental conditions such as dry spells, overcrowding, high or low temperatures, excessive organic matter, deteriorated water quality, among others stressors. These conditions affect fish by decreasing or changing their physiological capacities to respond against putative pathogens (Langdon et al. 1985, Hurst 2007, Noga 2010).
In the Paraná River basin, the construction of large dams, and the formation of vast reservoirs have triggered important changes in the environment (Araya et al. 2005, Agostinho et al. 2008, Bauni et al. 2015). These modifications include shifts in low and high-water phases, obstruction of migratory routes, and biotic homogenization (Agostinho et al. 2004, 2021, Poff et al. 2007, Bauni et al. 2015, Arantes et al. 2019). Additionally, recent years have witnessed notable changes in environmental conditions attributable to the increase in the global average temperature, accompanied by hydrological and meteorological anomalies (Berri et al. 2019, Bomfim et al. 2021, Mokhov 2022). These environmental modifications can affect the biotic and abiotic balance and, consequently, inducing stress effects on fish (Alfonso et al. 2021) and the occurrence of fish communities with greater vulnerability to diseases (Thomaz et al. 2007, Petsch 2016). In this context, fish parasites exhibit remarkable adaptability to diverse environmental conditions (Costa et al. 2021), possibly resulting in a problem on fish health (Lafferty and Kuris 1999, Schisler et al. 1999, Galli et al. 2001, Silva et al. 2011, Falkenberg et al. 2019), with potential mortal consequences (Langdon et al. 1985). In this context, chilodonelliasis emerges as a parasitic fish disease affecting fish devoid of host specificity and inflicting severe injuries on gills and tegument (Noga 2010, Bastos Gomes et al. 2017a). The occurrence of this disease has been linked to temperature fluctuations and alterations in water quality (Langdon et al. 1985, Schisler et al. 1999, Mitra and Haldar 2004, Hu 2012, Deng et al. 2015).
The aim of this report was to document a fish mortality occurred during a winter period at the confluence of the sub-reservoir of the Garupá and Pindapoy Grande streams (Misiones, Argentina).
Materials and methods
Area of study and environmental variables. The Garupá (27°26′49″ S 55°48′16″ W) and Pindapoy Grande streams (27°28′34″ S 55°48′25″ W) are located in a subtropical environment near the city of Garupá (Misiones, Argentina). Mortality was recorded at the confluence of the Pindapoy Grande stream with the Garupá stream, shortly before the mouth of the Yacyretá dam reservoir (Figure 1A).
The environmental parameters measured at the moment of the epizootic were as follows: environmental temperature 5 °C, water temperature 15.1 (±0.6) °C, dissolved oxygen
8.7 (±1.2) ppm, pH 7.5 (±0.2), electrical conductivity 67.6 (±8.8) mS cm-1 and mean depth of 1.7 (±1.0) m. However, two weeks before the epizootic event, the region experienced an environmental temperature nearing 1 °C, despite the average annual temperature being 23.6 °C and in August the average temperature is around 18 °C.
Fish and histopathological study. In August, during winter 2021, more than 150 dead and moribund fish were found on the water surface (Table 1). Most of the fish recorded in this episode presented characteristics of having died between 3 and 5 days, while the few moribund species were identified on the water surface.
Moribund fish (1-2 individuals of the most affected species) were captured and conditioned for histological examinations. Tegument scrapings and gill smears were performed to detect parasites presence under a LEICA EZ 4W magnifying glass. Samples were scraped onto slides, air-dried at room temperature, then Giemsa stained and impregned with 2% silver nitrate (Klein 1958). Subsequently, the gills and internal organs of fishes (digestive tract, liver, and kidney) were removed and fixed in Bouin’s solution for 24 h, then routinely processed for histology. Sections 3 µm thickness were cut from the samples and stained with hematoxylin and eosin. Cells measurements and characteristics were recorded with a Leica DM500 microscope and a LEICA ICC50 digital camera. Measurements were made using a Leica Application Suite 3.4.1 image analysis system, and the results are presented as the mean and ± standard deviation with the range in parenthesis.
Additional tegument and gill samples underwent dehydration at critical point and were subsequently coated with gold-palladium for analysis using a scanning electron microscope (SEM). The SEM observations were conducted using a JEOL5800LV SEM, housed within the Electronic Microscopy Service of the General Secretary of Science and Technology (UNNE).
Results
Mortality area and affected species. At the mouth of the Pindapoy Grande stream, dead and moribund fish were found, distributed mainly on the coastal margins of the sub-reservoir (Figure 1A). Of the 20 species recorded, only five (Potamorhina squamoralevis, Leporinus lacustris, Metynnis mola, Schizodon borellii and Steindachnerina conspersa), both juveniles and adults, were the main affected fish species. Although these species do not have an important economic value, they do have a great ecological importance in their environment.
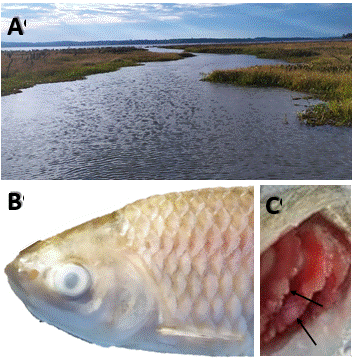
Figure 1 (A), Mouth of the Pindapoy Grande stream where dead and dying fish were found. (B), Moribund fish collected (L. lacustris) showing corneal opacity, as well as whitish integument. (C), Significant branchial alteration (whitish lesion; arrow).
Table 1 List of affected species and estimated number of dead fish
| Species | 61.Estimation |
|---|---|
| Acestrorhynchus pantaneiro | 64.x |
| Cyphocharax spilotus | 67.x |
| Geophagus sveni | 70.x |
| Gymnogeophagus balzanii | 73.x |
| Hoplias argentinensis | 76.x |
| Leporinus lacustris | 79.xxxx |
| Loricariichthys platymetopon | 82.x |
| Metynnis mola | 85.xxxx |
| Mylossoma duriventre | 88.xx |
| Plagioscion ternetzi | 91.xxx |
| Platydoras armatulus | 94.x |
| Potamorhina squamoralevis | 97.xxxx |
| Psectrogaster curviventris | 100.xxx |
| Roeboides descalvadensis | 103.xx |
| Roeboides microlepis | 106.xxx |
| Schizodon borellii | 109.xxxx |
| Steindachnerina conspersa | 112.xxxx |
| Tetragonopterus argenteus | 115.x |
| Trachydoras paraguayensis | 118.x |
| Triportheus nematurus | 121.x |
Note: Number of dead fish, (x) 1 to 2, (xx) 3 to 6, (xxx) 7 to 12, (xxxx) more than 12.
Gross and microscopic pathology. Macroscopic examination of moribund fish revealed corneal opacity, whitish integument, abundant mucus and whitish areas in gill (Figure 1B, C). Wet mount preparations of tegument scrapings and gill smears examined under a light microscope revealed numerous parasites. From the total of parasites found, the highest proportion (98%) was represented for ciliated protozoan forms, which were dorsoventrally flattened and with striations on their bodies, compatible with the genus Chilodonella. These were observed mainly on the gill and, to a lesser extent, in the tegument. In addition, other parasitic forms such as Ichthyophthirius multifiliis (1.2%), Dactylogyridae (0.6%) and Trichodinidae (0.2%) were observed. Likewise, the highest incidence was recorded in P. squamoralevis, followed by L. lacustris.
Histopathological examination revealed that the whitish areas observed macroscopically in the gill tissue corresponded to necrotic regions. Furthermore, tissue destruction was closely correlated with the presence of intense Chilodonella sp. infections in all moribund fish collected (Figure 2A).
Within each histological section, the parasites were situated between the gill lamellae, with a concentration towards the ends of the secondary lamellae (Figure 2B). Chilodonella sp. infection was higher in the P. squamoralevis specimens collected. However, in the rest of the examined species the parasite load was lower.
Heavy infections were associated with severe and generalized gill lesions, while species with moderate infections displayed minimal or no alterations. The main changes observed were gill lesions, characterized by extensive areas of epithelial hyperplasia and lamellar fusion, affecting multiple adjacent lamellae. In the most severe cases, identification of filaments with complete erosion of lamellae (Figure 2B), filament necrosis, a marked inflammatory response along the gill filaments and abundant eosinophilic granular cells (Figure 2C, D), were present in the primary lamellae of some, but not in all infected fish. In the tegument, inter-cellular oedema (spongiosis) was observed, related to the macroscopic observation of whitish integument. Alterations consisted of loss of contact between the epithelial cells. No other alterations related to the death of the fish were observed.
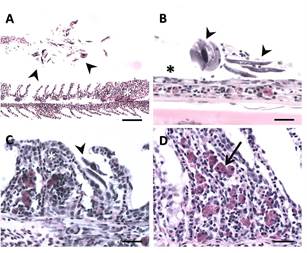
Figure 2. Gills of P. squamoralevis affected by Chilodonella sp. (A), Branchial filament with lamellar epithelial hyperplasia (white asterisk). Note numerous parasites (arrowheads) in proximity to the gill filament. Scale bar 131. = 100µm. (B), Primary gill filament devoid of secondary lamellae (asterisks), as well as numerous parasites on the filament (arrowheads). Scale bar = 25 µm. (C), Branchial filaments with synechiae of multiple adjacent gill lamellae (asterisk). Note numerous parasites (arrowheads). Scale bar = 25 µm. (D), Lamellar fusion and abundant presence of eosinophilic granular cells (arrow). Scale bar = 25 µm.
Chilodonellid description. Considering that the genus Chilodonella has not yet been described in Argentina, we find it pertinent to describe the main characteristics of this parasite encountered in the affected fish gills (Figure 3). These organisms were oval in shape and dorsoventrally compressed. Body dimensions were 53.6±8.2 µm (42-69 µm) long, 37.2 ± 5.4 µm (28-47 µm) wide. Ventral surface ciliated, with right and left ciliary rows separated by a sparse central non-ciliated zone. Large oval macronucleus and a single small micronucleus. Vacuoles of different sizes (Figure 3A). Kineties 10-12 on the left portion (short straight rows), 12-15 on the right portion (longer curved rows) (Figure 3B).
Discussion
The mortality episode occurred in the sub-reservoir of the Pindapoy Grande stream occurred during the dry season and may be linked to an exceptionally low flow of the Paraná River in 2021, coupled with extreme winter temperatures recorded during this period. Within the Paraná River basin, numerous instances of massive fish mortality have been documented, often associated with periods of dry season, low temperatures and deteriorating water quality (Freyre 1967, Mancini et al. 2000, González Naya et al. 2011, Gómez 2014). According to Hurst (2007), low- temperature stress in fish is generally responsible for the episodic mass mortalities. Additionally, González Naya et al. (2011), found that the likelihood of massive mortalities in a fish community increases when the central point of variation of the air temperature falls below the mean annual value, as observed in this instance. Consequently, it is plausible to surmise that the fish congregated in shallow areas, where the abrupt temperature drop reached critical levels causing injuries and making them susceptible to potential opportunistic organisms. Subsequently, they may have been carried by the stream´s current to the mouth of the sub-reservoir, where the presence of dead and moribund specimens was noted. Recent years have a rise episodes of low water levels and extreme droughts across the Paraná River basin (Naumann et al. 2021, Santos et al. 2021), with the alteration of global temperature considered one of the foremost factors driving modification in freshwater systems (Berri et al. 2019). These changes jeopardize the natural diversity of these ecosystems (Andreotti et al. 2021, Marengo et al. 2021).
The histopathological study carried out on moribund fish allowed the identification of different types of parasites, with the ciliate Chilodonella exhibiting an intense parasitosis. In Brazil, reports of massive fish mortalities linked to this ciliate have been documented in aquaculture settings (Franceschini et al. 2013, Padua et al. 2013, Valladão et al. 2019, Wang et al. 2019), along with its presence in wild fish populations (Silva et al. 2011, Carvalho et al. 2021). Therefore, our finding provides valuable information, as there are no prior records of epizootic cases associated with Chilodonella infection in either wild or farmed fish populations. This marks the first report of chilodonelliasis for this region.
In this scenario, the decline in water temperature may have facilitated the proliferation and spread of Chilodonella, which exhibits rapid multiplication at temperatures below 15 °C (Ogut and Akyol 2007, Padua et al. 2013, Bastos Gomes et al. 2017a). This lends support to the hypothesis that abrupt changes in environmental conditions compromise the physiological resilience of fish against diseases (Langdon et al. 1985), while creating a conducive environment for these ciliates’ reproduction (Bastos Gomes et al. 2017a). Langdon et al. (1985) reported massive winter mortality in the Finke River (Australia), attributed to severe parasitosis caused by C. hexasticha, suggesting that low winter water temperatures diminish the ability of fish to fend of infections. In the present report, the histopathological alterations observed in the gills revealed epithelial hyperplasia of the branchial filament, lamellar fusion and necrotic regions of the branchial tissue. Moreover, a pronounced immune response was noted in the gills of the parasitized fish, consistent with findings in Oncorhynchus mykiss (Schisler et al. 1999), Nandus nandus (Mitra and Haldar 2004), Piaractus mesopotamicus (Padua et al. 2013), Lates calcarifer (Bowater and O’Donoghue 2014), and Astyanax lacustris (Valladão et al. 2019). The severity of the lesions likely led to a reduction in gas exchange, respiratory collapse, and subsequent death of the infected fish (Valladão et al. 2019), consistent with a chronic mortality pattern (Urawa and Yamao 1992, Bowater and O’Donoghue 2014). However, in chronic episodes, fish often develop infections associated with other pathogens simultaneously, such as bacteria or fungi. Although other ectoparasites were detected in the sampled fish, the high 6. prevalence of Chilodonella suggest that mortality may be linked to this ciliate protozoan. Additionally, no other potential causes of mortality were identified through histopathological examinations conducted on affected fish.
The morphometric analysis conducted on the parasite, based on body measurements and ciliary row counts, suggest a relationship with the species as C. piscicola, consistent with previous studies (Wiles et al. 1985, Urawa and Yamao 1992, Ogut and Akyol 2007, Abdel-Baki et al. 2014, Bastos Gomes et al. 2017a). The total number of ventral kineties serves as key characteristic for species identification and differentiation with C. hexasticha (Kazubski and Migala 1974), having less than 14 kineties and C. piscicola having more than 21 (Wiles et al. 1985). However, discrepancies may arise due to morphological variations during different life cycle stages or environmental influences (Bastos Gomes et al. 2017b). Therefore, the morphological differentiation between these two species remains questionable (Abdel- Baki et al. 2014).
This report presents a new record of fish mortality related to extreme environmental conditions and parasitosis, providing valuable information to understand how extreme environmental conditions impact negatively affecting fish communities. These types of report are instrumental in informing the management and implementation of conservation strategies in areas subject to varying degrees of anthropogenic impacts (Thomaz et al. 2007, Agostinho et al. 2008, Petsch 2016, Costa et al. 2021).
















