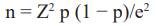Introduction
In households in West African countries, small ruminant farming is an important source of income. The demand for animal protein has increased in the Republic of Benin over the past two decades due to demographic changes, which have significantly increased the level of numerical productivity of society (Mensah et al. 2017). However, this early development of small ruminants continues to be plagued by several problems related to infectious diseases (bacterial, parasitic, and viral) with public health implications, such as quinine fever disease, campylobacteriosis, toxoplasmosis and, most significantly, enzootic sheep abortion (Sidibe et al. 2019). Ovine Enzootic Abortion is a disease caused by a very formidable etiological agent called Chlamydia abortus (C. abortus) formally known as C. psittaci serotype 1. It is a non-motile, coccoid, and obligate intracellular parasite and belongs to the family Chlamydiaceae, which has recently been reclassified and now comprises 11 distinct species (Schnee 2014).
C. abortus is one of the main causes of reproductive failure in sheep and goats (Sachse et al. 2005). In sheep, the disease usually manifests itself as an abortion in the last 2-3 weeks of gestation, while goats can abort at any stage of pregnancy, but most abortions occur in the last 2-3 weeks of gestation (Osman et al. 2011).
The disease was first detected in West Africa especially in Mali with an overall prevalence of 3,55%, in Morocco with a prevalence of 15,09% and 27,23% in sheep and goats, and in Ethiopia with a prevalence of 9,88%. Furthermore, blood hematological indicators, which vary according to many circumstances, are the main predictor of an animal’s adaptability to its environment (Sattar and Mirza 2009).
These parameters are influenced by many factors and may even differ according to breed and physiological state, particularly in animals infected with C. abortus. However, the data available on the issue in the sub-region in general and in the Republic of Benin, in particular, are patchy (non-confirmation of the disease in all countries due to the “neglected” character of this zoonosis). The aim of this study is to determine for the first time the serological prevalence and hematological parameters in small ruminants positive for chlamydia abortus mainly in the governorate of Ouémé in Benin Republic.
Materials and methods
Period and type of study. This is a cross-sectional survey with a descriptive aim, conducted during the period from October 2022 to June 2023.
Study environment. The study took place in the governorate of Ouémé, in the south of Benin. This governorate includes the communes of Adjara, Akpro- Missérété, Avrankou, Adjohoun, Bonou, Dangbo, Sèmè- Kpodji and Aguégués, but only 5 of the 9 communes in the Ouémé governorate were surveyed. These communes were Adjara, Akpro-Missérété, Avrankou, Dangbo and Sèmè-Kpodji. The animals were Djallonké ewes and goats. However, the various communes included in the study were chosen on the basis of the number of animals held, according to the 2021 final report of the Agricultural Statistics Directorate.
Study design. During 2022-2023, a cross-sectional study was conducted with owner’s consent, approved by the Ethics Review Committee of the University of Abomey- Calavi. Sample size was calculated using Cochran’s formula:
where n is the sample size, Z is the statistic for 95% confidence interval, p is expected prevalence (50% due to Benin Republic’s unaffected status regarding C. abortus infection), and e is precision (5%). 385 samples were collected from 18 sheep flocks in five municipalities of Ouémé governorate, Southern Benin. Samples were randomly collected from both species (200 ewes and 185 goats) aged between <2 to >3 years, across four seasons. Some examined animals had a history of abortion (n = 345). Additionally, some examined flocks had lambing pens (n = 355) used as a clean area for parturition, and some implemented hygienic measures after abortion, such as isolating infected animals or disinfecting contaminated pens.
Sample collection and preparation. A suction tube was used to collect approximately 5 mL of blood from each animal’s jugular vein. The sera were isolated from the clotted blood by centrifugation at 3000 rpm for 05 minutes and stored at 20 degrees Celsius until serological analysis.
Laboratory analysis
Serological analysis. Diagnostic work done at Bohicon Veterinary Laboratory’s serology unit using IDVET’s (Montpellier, France) indirect ELISA kits for Chlamydia abortus chlamydiosis. Tests followed manufacturer’s guidelines and were read at 450 nm using Chromate Inc’s ELISA reader. Validation criteria for this zoonotic disease: mean optical density of positive controls > 0.350 and mean optical density ratio (DO pc/OD nc) > 3.
Hematological analysis. Animals were separated and fasted the day before blood sampling. Blood was collected from tiny ruminants using a single-use needle in their jugular or saphenous veins. The blood was stored in a labeled tube with a red cover, placed in an ice-filled fridge, and sent for analysis at the Bohicon veterinary laboratory. The hematological examination was done using the Sysmex XN-Series automated device. Thirty chlamydia abortus-positive sheep and goats were sampled to assess the disease’s impact on their blood parameters.
Statistical Analysis. Study data analyzed using SPSS V17 (IBM) and Student t-test. Logistic regression assessed risk factors’ association with Chlamydiosis. Means and standard deviations for hematological parameters computed. One-way ANOVA tested hematological measures relationship with variables: animal locations, age, species, and physiological stage. If p-value <0.05, difference considered significant.
Results
Seroprevalence and factors associated with C. abortus infection. The prevalence of C. abortus was studied in small ruminants in five municipalities in southern Benin. The results showed significant differences between the different areas at p<0.05, with the highest prevalence observed in the municipality of Akpro-Missérété followed by the municipality of Sèmè-Kpodji (Table 1).
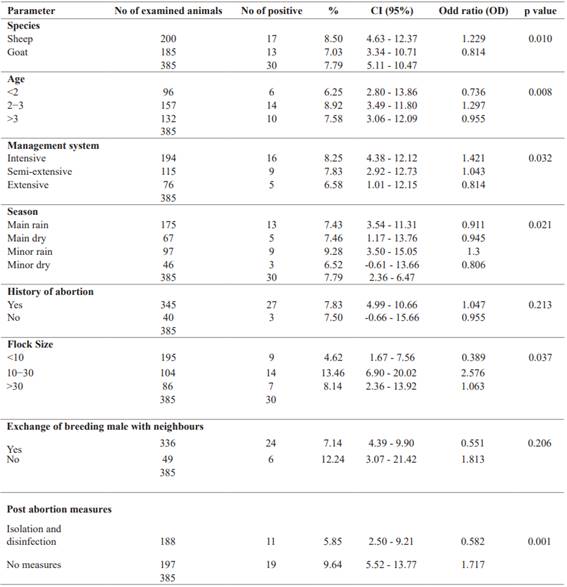
Variables associated with the prevalence of C. abortus infection were assessed using the logistic regression method (Table 1). Seropositivity was significantly (p = 0.010) higher in ewes than in goats. Interestingly, season played a significant role (p = 0.021) in the prevalence of C. abortus.
The prevalence of C. abortus infection in small ruminants was higher during the short rainy season and the long dry season than during the long rainy season and the short dry season. The results obtained did not show a strong relationship between abortion and C. abortus infection (p = 0.213), with the seropositivity rate being highest in animals with a history of abortion.
In terms of management system on the farms examined, the prevalence rate was highest on the farm where the animals had been aborted and where animals were raised intensively and significant (p = 0.032). The prevalence rate was significantly higher on farms where the herd size was between 10 and 30 animals or where no post-abortion hygiene measures were applied. On the other hand, the abortion history of the animals examined and the number of breeding males that could be exchanged with neighbors had no significant effect on the prevalence of C. abortus infection (Table 2).
Table 1: Prevalence of C. abortus infection across the sites and around World and Regions (Kifouly et al. 2023).
| 65. Parameter | 66.No of examined animals | 67.68. No of positive | 69.70. % | 71.72. CI (95%) | 73.74. p value | 75.Odd ratio (OD) | 76.Prevalence by world and regions | 77.78. % |
|---|---|---|---|---|---|---|---|---|
| Sèmè-Kpodji | 81.85 | 82.9 | 83.10.59 | 84.4.05 - 17.13 | 85.86. | 1,573 | 87.Worldwide | 88.13.4 |
| Akpro-Missérété | 91.90 | 92.11 | 93.12.22 | 94.5.46 - 18.99 | 95.96. | 2,022 | 97.Regions | 98.|
| Dangbo | 101.70 | 102.5 | 103.7.14 | 104.1.11 - 13.18 | 105.1,28E-05 | 106.0,892 | 107.Africa | 108.9.1 |
| Adjarra | 111.65 | 112.3 | 113.4.62 | 114.0,49 - 9.72 | 115.116. | 0,525 | 117.Asia | 118.48.5 |
| Avrankou | 121.75 | 122.2 | 123.2.67 | 124.0,98 - 6.31 | 125.126. | 0,276 | 127.Europa | 128.33.3 |
| Total | 131.385 | 132.30 | 133.7.79 | 134.5.11 - 10.47 | 135.136. | 137. | North and South America | 138.3 6.1 |
* The result is significant at p<0.05.
*The result is significant at p<0.05
Table 2: Variables associated with Chlamydia abortus infection in small ruminants
Site-specific assessment of hematological parameters. Hematological data of C. abortus -positive small ruminant samples from various sites are shown in Table 3. This indicating possible blood composition differences between sites. Hemoglobin varied greatly, with the highest in Adjarra and lowest in Avrankou. Hematocrit remained relatively constant. Mean Corpuscular Volume were lowest at Akpro-Missérété and highest at Adjarra. Mean corpuscular hemoglobin varied with significant site differences.
Table 3: Site-specific values in hematological parameters of red blood cells
| Sites 149. 150. 151. | 152. 153.|||||||
|---|---|---|---|---|---|---|---|
| 154. Parameters | 155.Adjarra | 156.Akpro- Missérété | 157.Avrankou | 158.Dangbo | 159.Sèmè- Kpodji | 160.161. p-value | 162.Reference by Research Animal Resources. 2009 | 163. 164.
| 165. | Means ± SD | 166.Means ± SD | 167.Means ± SD | 168.Means ± SD | 169.Means ± SD | 170.171. 172. 173. | |
| Red Blood (106/mm3) | 174.175. 4.67±0.77 | 176.177. 4.72±0.85 | 178.179. 3.30±0.00 | 180.181. 3.82±0.34 | 182.183. 3.26±0.16 | 184.185. 0.479 | 186.187. 8 - 15 | 188. 189.
| White Blood (103/mm3) | 190.191. 5.61±0.85 | 192.193. 6.47±3.12 | 194.195. 7.90±0.00 | 196.197. 7.46±1.47 | 198.199. 7.83±0.17 | 200.201. 0.844 | 202.203. 4 - 12 | 204. 205.
| Hemoglobin 206. (g/dl) | 207.208. 16.70a±0.20 | 209.210. 9.33b±0.69 | 211.212. 7.30b±0.02 | 213.214. 11.40b±2.48 | 215.216. 9.88b±1.13 | 217.218. 0.001 | 219.220. 8 - 16 | 221. 222.
| Hematocrit% | 223.14.50±0.20 | 224.13.97±0.76 | 225.13.70±0.00 | 226.14.50±1.80 | 227.12.65±1.25 | 228.0.731 | 229.24 - 45 | 230. 231.
| MCV (fl) | 232.48.87a±0.13 | 233.26.06c±2.77 | 234.48.70a±0.15 | 235.41.53ab±3.34 | 236.33.18bc±3.81 | 237.0.001 | 238.23 - 48 | 239. 240.
| MCH (pg) | 241.32.33±0.17 | 242.28.23±1.45 | 243.33.00±0.00 | 244.29.03±0.12 | 245.33.18±3.81 | 246.0.100 | 247.8 - 12 | 248. 249.
| MCHC(g/dl) | 250.94.77±0.97 | 251.79.90±4.08 | 252.97.50±0.00 | 253.91.87±2.98 | 254.84.70±8.06 | 255.0.104 | 256.31 - 38 |
p<0.05 = significant difference; p>0.05 = Not significant difference, SD: Standard Deviation
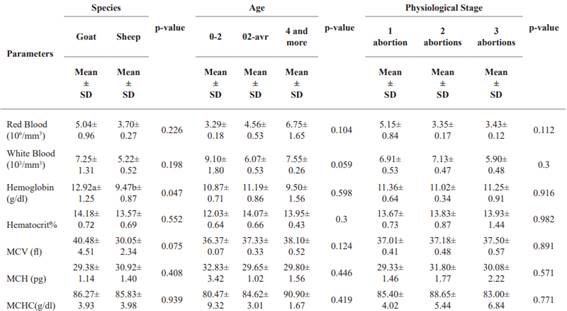
Assessment of hematological parameters of the red blood cells as a function of species (Djallonké goats and sheep), age and physiological stage of infected small ruminants. Table 4 compares hematological parameters by species (Djallonké goats and sheep), age, and physiological stage. Goats generally had higher RBC and WBC counts than sheep, but the differences weren’t significant. Goats had significantly higher Hb levels tan sheep, while hematocrit showed no significant difference. RBCs increase with age, while WBCs decrease slightly. Hemoglobin values are highest in the 2-4-year age group, and hematocrit increases with age. No clear trend for VCM, HCM, and CCMH based on age. Red blood cell counts slightly decreased with more abortions, but the differences weren’t significant. Other hematological parameters didn’t significantly vary with physiological stage.
Evaluation of white blood cell hematological parameters according to site. Table 4 shows white blood cell line evaluations for different locations. Neutrophils had the highest mean at Akpro-Missérété and the lowest mean at Avrankou, but this difference wasn’t statistically significant (p = 0.100). Lymphocytes had the highest mean at Sèmè-Kpodji and the lowest mean at Avrankou and this difference was statistically significant.
Table 6: Site-specific values in hematological parameters of the white blood cells
| 270. | 271. | 272. | Sites | 273.274. | 275. | 276. | Reference by Research Animal Resources. 2009 | 277. 278.
|---|---|---|---|---|---|---|---|
| 279. | Adjarra | 280.Akpro- Missérété | 281.Avrankou | 282.Dangbo | 283.Sèmè-Kpodji | 284.p-value | 285. 286. 287.|
| Parametres | 288. 289. 290. 291. 292. 293.294. 295. 296. | ||||||
| 297. | Mean ± SD | 298.Mean ± SD | 299.Mean ± SD | 300.Mean ± SD | 301.Mean ± SD | 302.303. 304. 305. | |
| Neutrophils % | 306.35.80±2.54 | 307.40.14±2.68 | 308.35.60±0.00 | 309.37.10±2.08 | 310.48.03±3.39 | 311.0.100 | 312.25 - 75 | 313. 314.
| Lymphocytes% | 315.16.40b±1.85 | 316.18.30ab±0.99 | 317.12.00b±0.85 | 318.17.43b±0.46 | 319.16.23a±2.33 | 320.0.049 | 321.40 - 70 | 322. 323.
| Monocytes(%) | 324.6.90±0.17 | 325.3.17±1.23 | 326.6.80±0.00 | 327.2.90±1.78 | 328.2.55±1.32 | 329.0.061 | 330.0 - 6 | 331. 332.
| Eosinophils(%) | 333.15.25±3.49 | 334.17.44±3.07 | 335.25.50±0.00 | 336.10.97±3.40 | 337.10.75±2.83 | 338.0.070 | 339.0 - 10 | 340. 341.
| Basophils (%) | 342.5.70c±0.29 | 343.14.91b±2.23 | 344.10.80bc±0.15 | 345.4.93c±1.95 | 346.22.45a±0.82 | 347.0.001 | 348.0 - 3 |
p<0.05 = Significant difference; p>0.05 = Not significant difference, SD: Standard Deviation
Evaluation of hematological parameters of the white lineage as a function of species (Djallonké goats and sheep), age and physiological stage of infected small ruminants. Table 7 presents data on white blood cell parameters in Djallonké goats and sheep, according to species, age and physiological stage. Neutrophil levels were similar between the two species, while lymphocyte, monocyte, eosinophil and basophil levels showed slight but non-significant variations. With regard to age, lymphocytes increased significantly, while the other blood parameters showed no significant differences. With regard to physiological stage, neutrophils and eosinophils remained stable, while lymphocytes, monocytes and basophils showed significant differences, indicating variations according to the number of abortions.
Table 7: Values in white blood cell parameters as a function of species, age and physiological stage
| Species 356. 357. | 358.359. | 360. | Age | 361.362. | 363. | 364. | Stade Physiological 365. | 366.367. | 368. 369.||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 370. Goat Sheep 371. | 372.p-value | 373.374. 0-2 | 375.376. 02-avr | 377.4 | 378.p-value | 379.1 | 380.2 381. | 382.3 | 383.p-value | 384. 385.|||
| Parameters | 386.387. | 388. | 389. | 390.391. | and more | 392.abortion 393. | 394.abortions | 395.abortions 396. 397. | 398. 399.||||
| 400. | Mean | 401.Mean | 402.Mean 403. | 404.Mean | 405.Mean | 406.Mean 407. | 408.Mean | 409.Mean 410. 411. | 412. 413.||||
| 414. | ± | 415.± | 416.± 417. | 418.± | 419.± | 420.± 421. | 422.± | 423.± 424. 425. | 426. 427.||||
| 428. | SD | 429.SD | 430.SD 431. | 432.SD | 433.SD | 434.SD 435. | 436.SD | 437.SD 438. 439. | 440. 441.||||
| Neutrophils 442. % | 443.16.05± 444. 1.21 | 445.17.65± 446. 1.02 | 447.0.331 | 448.19.83± 449. 1.17 | 450.16.29± 451. 0.92 | 452.15.90± 453. 1.20 | 454.0.199 | 455.16.10± 456. 1.57 | 457.16.25± 458. 0.61 | 459.19.97± 460. 0.84 | 461.0.117 | 462. 463.|
| Lymphocytes 464. % | 465.36.03± 466. 1.44 | 467.41.58± 468. 2.31 | 469.0.059 | 470.36.03± 471. 1.53 | 472.40.94± 473. 1.93 | 474.53.60± 475. 2.60 | 476.0.021 | 477.38.63ab± 478. 1.50 | 479.46.18a± 480. 3.78 | 481.36.03b± 482. 1.08 | 483.0.045 | 484. 485.|
| Monocytes (%) | 486.3.93± 487. 1.32 | 488.3.4± 489. 0.91 | 490.0.747 | 491.4.30± 492. 1.85 | 493.3.32± 494. 0.87 | 495.1.35± 496. 0.05 | 497.0.553 | 498.5.17a± 499. 0.99 | 500.0.85b± 501. 0.18 | 502.5.10a± 503. 1.53 | 504.0.008 | 505. 506.|
| Eosinophils (%) | 507.16.68± 508. 3.91 | 509.14.35± 510. 1.84 | 511.0.605 | 512.12.13± 513. 1.05 | 514.16.71± 515. 2.37 | 516.5.95± 517. 0.35 | 518.0.163 | 519.15.69± 520. 2.58 | 521.16.05± 522. 4.37 | 523.11.97± 524. 0.76 | 525.0.692 | 526. 527.|
| Basophils (%) | 528.8.73± 529. 2.35 | 530.14.27± 531. 2.37 | 532.0.12 | 533.7.03± 534. 2.52 | 535.14.98± 536. 2.19 | 537.23.20± 538. 1.10 | 539.0.065 | 540.11.13b± 541. 2.89 | 542.20.67a± 543. 1.07 | 544.7.33b± 545. 1.80 | 546.0.004 | |
p<0.05 = Significant difference; p>0.05 = Not significant difference, SD: Standard Deviation
Discussion
Seroprevalence and risk factors for C. abortus. The overall seroprevalence of C. abortus in unvaccinated goats and sheep was 7.79% (30 out of 385), while 89.61%
(345 out of 385) were negative and 2.60% (10 out of 385) produced suspect results. The variation in prevalence by commune ranged from 2.67% to 12.22% (see Table 2), and seropositive goats were identified in all sampled non-vaccinated herds. This finding suggests that the bacterium is widespread in the Ouémé governorate, posing a potential risk to humans and animals in direct contact or in the surrounding areas. In Africa, data on sheep and goat prevalence of enzootic abortion remain scarce; however, there are reports of significantly lower prevalence levels, including 7.10% recorded in the Republic of Mali (Sidibe et al. 2019). In Europe, the prevalence remains higher, exceeding the 5.8% observed in herds with recent cases of abortion in Sardinia (Masala et al. 2005), as well as 3.97% in Belgium (Yin et al. 2014) and 7.70% in the Slovak Republic (Čislákován et al. 2007).
The risk factors associated with seropositivity are shown in Table 3. Statistically significant associations were identified for herd size, animal species, age of animals, season, and post-abortion measures. With regard to herd size, statistical disparities were found between small and medium-sized herds compared with all herds, respectively. For example, the odds ratio (OR) for the presence of antibodies in goats from medium-sized herds was almost twice as high (OR= 2.576; CI = 6.90 - 20.02; p = 0.037) as in goats from small or large herds. Conversely, goats from small herds had an OR of 0.39 (CI = 1.67 - 7.56), a protective characteristic. Herd size was related to the animal management methods used on farms; most herds were subject to intensive or semi- extensive management practices, favoring close contact between animals, which could facilitate transmission of the bacteria and spread of the disease (Campos-Hernández et al. 2014). In this context, seroprevalence was found to be significantly higher in intensively managed herds (OR = 1.421; CI = 4.38 - 12.12; p = 0.032) compared with those under semi-extensive or extensive management (Mamlouk et al. 2020). In addition, inappropriate management practices have also been associated with a high incidence of zoonotic abortions within herds (Lenzko et al. 2011).
Significant seasonal variations have been observed. Specifically, a statistically significant significance was noted during the season of heavy rainfall (OR = 0.911; CI = 3.54 - 11.31; p = 0.021), even though a higher incidence of infections was observed during the small rainy season (9.28%). In fact, the risk of infection in animals was nearly 1.5 times higher (OR = 1.301; CI = 3.50 - 15.05) during this period than during other seasons. The rainy season promotes an increase in food availability and, consequently, creates favorable environmental conditions for the proliferation of C. abortus (Essig and Longbottom 2015). Positive cases observed during the dry season can be explained by factors such as food scarcity, long distances between grazing and shelter areas, exposure to high temperatures, and a shortage of clean water, common conditions during this period that can induce stress and lead to abortions (Sánchez-Rocha et al. 2021). The season was also associated with measures of prevention taken after abortions. A high prevalence (9.64%) was observed in farms where no preventive measures were in place, with an infection risk almost two times higher (OR = 1.717; CI = 5.52 - 13.77) compared to farms where preventive measures were partially in effect (OR = 0.582; CI = 2.50 - 9.21). Herd disinfection and cleaning of contaminated equipment reduce the risk of disease spread in the livestock (Robertson et al. 2018) and eliminate potential pathogens dispersed in the environment by seemingly healthy animals (Zezekalo et al. 2020). Regarding species-specificity, a significantly higher prevalence (8.50%) was observed in sheep compared to goats, with sheep being 1.2 times more likely to be infected than goats (OR = 1.229; CI = 4.63 - 12.37; p = 0.010). This phenomenon can be attributed to the greater susceptibility of sheep to C. abortus compared to goats (Selim 2016; Seth-Smith et al. 2017). Furthermore, small ruminants aged 2 to 3 years exhibited a notably higher prevalence (8.92%) than individuals in other age groups (<2 years and >3 years), with these animals being 1.3 times more likely to be infected than their counterparts in different age groups (OR = 1.297; CI = 3.49 - 11.80). This trend can be explained by the gradual acquisition of natural immunity in animals as they grow, providing protection against infectious diseases such as C. abortus (Casanova and Abel 2021).
Hematological parameters of infected C. abortus small ruminants. Knowledge of the reference values for blood during gestation and lactation helps the veterinarian in the diagnosis, prognosis and treatments used to improve ewe production and reproduction (Roubies et al. 2006).
The results presented in Tables (3 and 4) show that the mean GR (ranging from 3.26 × 106/ mm3 to 4.72 × 106/ mm3 at site level; 5.04 × 106/ mm3 and 3.70 × 106/ mm3 in goats and sheep; ranging from 3.29 to 6.75 × 106/ mm3 at age level; ranging from 3.35 to 5.15 × 106/ mm3 at physiological stage) obtained in the Djallonké breeds studied is lower than the reference value reported (Research Animal Resources 2009). These results are also lower than those obtained in ewes at the end of pregnancy in the Santa Inês and Morada Nova breeds in Brazil (Bezerra et al. 2017), in Turkey (Cihan et al. 2016), in Nigeria (Adeyeye and Ate 2017) and in Algeria (Aiche et al. 2020). This decrease in RBC value in small ruminants that have had abortions could be due to the decrease in red blood cell production in the bone marrow leading to inflammatory anemia following infection with C. abortus (Waziri et al. 2010).
In addition, no non-significant variation in WBCs was observed across sites (ranging from 5.61 to 7.90 × 103/ mm3), age (5.22 to 7.25 × 103/ mm3), species (ranging from
6.07 to 7.55 × 103/ mm3) and physiological stage (ranging from 5.90 to 7.13 × 103/ mm3) of the animals. These values are within the range of (Research Animal Resources 2009). The stress that animals undergo during gestation stimulates the secretion of certain hormones that can increase hematopoietic activity and the movement of blood cells in the circulatory system, thereby increasing the number of WBCs (Dellmann et al. 1987).
A slight significant variation in Hb at site level (ranging from 7.30 g dl-1 to 16.70 g dl-1) and at species level (9.47 g dl-1 in sheep and 12.92 g dl-1 in goats) but remains constant at age and physiological stage. These values are within normal ranges (Research Animal Resources 2009) but lower than those obtained by (El-Malky et al. 2019) in pregnant Ossimi and Barki breeds, and (Plaza Cuadradro et al. 2019), but different from those reported by (Bezerra et al. 2017) and (Adeyeye and Ate 2017), who recorded higher values of HB in ewes at the end of pregnancy. This observation can be explained by the fact that C. abortus can affect the production and survival of red blood cells, leading to a reduction in their number, and that the capacity of red blood cells to transport oxygen is not altered. This would result in the presence of normochromic anemia induced by C. abortus.
Significant variation in MCH was observed at sites ranging from 26.06 to 48.87 fl and remained constant within the normal range for species, age and physiological stage (Research Animal Resources 2009). A considerable increase in MCH (ranging from 28.23 to 33.18 pg at the sites) and MCHC (ranging from 79.90 to 97.50 g dl-1) was observed at the sites as well as for the other parameters, and was significantly higher than in (Research Animal Resources, 2009). The MCH and MCHC values recorded in this study were higher compared to those obtained by (El-Malky et al. 2019) in ewes in the last third of their pregnancy; (Cihan et al. 2016) and (Bezerra et al. 2017) and (Tshiasuma et al. 2017). This increase in MCH and MCHC may indicate an increased concentration of hemoglobin in the red blood cells, which may be related to a response to oxidative stress or inflammation (Nazifi et al. 2005). MCH, which measures the size of red blood cells, can vary in response to a variety of factors, including inflammation and infection. In addition, the values of MCV and MCHC are essential transport markers of oxygen, necessary for cell survival. The increased oxygen demand stimulates an adaptive response in which increasing hemoglobin concentrations result in higher levels of oxygen transport (Gravena et al. 2010). All these results can be justified by the fact that when an ewe or goat is infected with C. abortus, the bacterium multiplies in the cells of the uterine mucosa and can cause tissue inflammation and necrosis. This can lead to abortion or the birth of weak young (Schnee 2014). In terms of the impact on the immune system, C. abortus infections can cause an inflammatory response in the infected animal. Prolonged inflammation can lead to a redistribution of immune cells, including neutrophils and lymphocytes, which are important white blood cells in the inflammatory immune response.
Conclusion
This study established the presence of the aetiological agent of enzootic ovine abortion disease in small ruminants in Ouémé department, the antibodies being of suspicious origin (animals not vaccinated against the disease studied). Seroprevalence rates varied according to study sites (Chlamydia rate higher in Akpro-Missérété, Sèmè-Kpodji and Dangbo), type of reproductive loss (predominance of positive cases in aborted animals), small ruminant species (sheep more infected with Chlamydia). In animals infected with C. abortus, significant variations in hematological parameters of the red and white bloodlines were observed, including differences according to study sites, species, age and physiological stage of the animals.