We report the case of a 79-year-old male patient, smoker, with hypertension, type 2 diabetes, coronary artery bypass surgery in 2009 due to chronic coronary syndrome, and prostatic adenocarcinoma actively followed-up by the Urology Department. The patient was referred to the Emergency Room due to dyspnea on moderate exertion for three weeks and right pleural effusion seen in a chest X-ray. Upon arrival, the patient was hemodynamically stable (blood pressure 153/93 mmHg, heart rate 99 bpm), and oxygen saturation at room air of 89%, with no tachypnea at rest. Physical examination revealed jugular venous disten tion up to the middle third of the sternocleidomas toid muscle, abolition of the vesicular murmur in the right lung base, and bilateral pitting edema up to both knees.
ECG showed sinus rhythm (89 bpm) with negative T-waves in V1-V4, already present in previous studies. Blood screening showed normal renal function (urea 31 mg/dL, creatinine 0.77 mg/dL, glomerular filtra tion rate 86 ml/min/1.73 m2) with all ions in range, C-reactive protein 25.98 mg/L, lactate dehydrogenase 950 U/L, creatinine kinase 55 U/L, NT-proBNP 950 pg. /mL, ultrasensitive troponin T 25 ng/L, hemoglobin 11.2 g/dL, platelets 335000/mm3, white blood cells 11430/mm3, and D-dimer 4860 ng/mL. To complete the diagnosis, the unsynchronized computed tomography (CT) scan revealed a hypodense lesion over the right cardiac chambers (Figure 1). Neoplasia or hemopericardium due to cardiac rupture or bypass dehiscence were suggested as first possible diagnosis. In view of these findings, evaluation by the Cardiology Department was requested. Transthoracic echocardiography (TTE) showed a heterogeneous, solid mass in the anterior pericardial sac, with adhesions in the right chambers and compression of the right atrioventricular sulcus, which did not capture echocardiographic contrast (Figure 2). The picture did not suggest a cardiac rupture, not only because of the echocardiographic findings, but also because the patient did not experience chest pain and was hemodynamically stable, and ECG did not show abnormalities suggestive of acute ischemia, making bypass dehiscence unlikely.

Fig. 1 CT scan. Top: Unsynchronized CT scan, with a hypodense lesion over the right cardiac chambers and severe right pleural effusion with associated collapse. Bottom: Synchronized CT scan, with a right precardiac mass of 8.5 x 10 cm, enhanced after contrast injection. The tumor exerts mass effect and possibly infiltrates the right atrium, in contact with anterior chest wall and possible infiltration of the right ventricle.
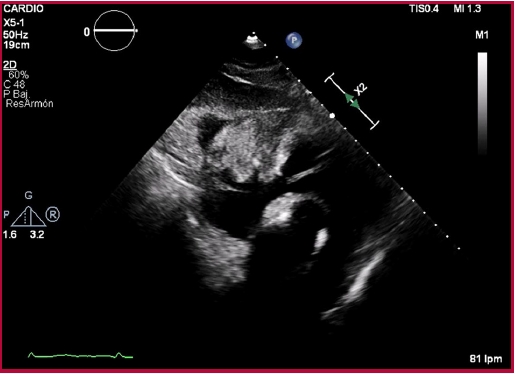
Fig. 2 Transthoracic echocardiography, subcostal access. Heterogeneous, solid mass in the anterior pericardial sac, compressing the atrioventricular groove and appearing to adhere to the ventricular wall.
Given the discrepancies, a synchronized CT scan was performed for better characterization of the lesion, which showed an 8.5 x 10 cm right precardiac mass, apparently depending on the pericardium, and showing enhancement after intravenous contrast (Figure 1). These findings raised the differential diagnosis of metastasis or primary pericardial neoplasia.
The differential diagnosis of mediastinal masses is primarily based on the location of the mass, its composition and the age of the patient. 1 Different radiological techniques, including CT scan and cardiac magnetic resonance (CMR), are of significant diagnostic value. Considering that the lesion was located in the anterior wall of the heart, in contact with the anterior chest wall, a broad differential diagnosis includ ing the different lesions at the level of the anterior mediastinum and the tissue-dependent masses in the pericardium was proposed.
Unlike in our patient, thymomas appear as an oval, homogeneous mass, with well-defined contours in CT scan. Calcifications and cystic areas are usually present in thyroid goiters and teratomas, 1-2 and considering that CT findings showed no calcifications in our patient, these two entities seemed unlikely. Lymphomas account for 20% of mediastinal tumors in adults, and Hodgkin's lymphomas are the most common subtype. 1-3 Within this subtype, mediastinal large B-cell lymphoma constitutes an independent entity within the classification of malignant lymphoid neoplasms, with a frequency estimated at 2-3% of non-Hodgkin's lymphomas and between 6-10% of large B-cell lymphomas. This tumor usually occurs as a rapidly expanding mediastinal mass and may be associated with pleural or pericardial effusion. 4,5
The patient was admitted to the Cardiology Department to complete assessment. An ultrasound-guided thoracentesis of the pleural effusion was performed, and a serosanguineous fluid consistent with an exudate (Light's criteria) was obtained, showing a hypercellular area in the cytological examination, with characteristics indicative of a B lymphoproliferative process. To complete the study, a core biopsy of the mediastinal mass was performed, confirming the diagnosis of primary mediastinal large B-cell lympho ma. Finally, the first cycle of chemotherapy was start ed with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine, and prednisolone. He is still on treatment.














