INTRODUCTION
In apparently healthy individuals, the risk of a future cardiovascular event is related to the number and intensity of risk factors and the duration of exposure. 1
The best known strategy in cardiovascular prevention is to use risk scores to identify those individuals at high risk of developing cardiovascular disease and to recommend preventive measures according to the calculated risk level. 1,2 Risk scores or risk functions are mathematical equations that calculate the probability that an individual will develop the event of interest within a specific period of time, according to the level of exposure to different risk factors. 3 However, these scores have major limitations related to calibration, discrimination and low sensitivity, as most cardiovascular events occur in the population group with low or intermediate risk. 4
Several additional biomarkers have been evaluated to improve cardiovascular risk stratification (risk modulators). 5 Detection of carotid atherosclerotic plaques (CAP) improves the prediction of cardiovascular events over and above that provided by models that only consider conventional risk factors. 6,7 Coronary artery calcium (CAC) score is associated strongly and in a graded fashion with the risk of presenting cardiovascular events, independently of age, sex, ethnicity, and traditional risk factors. 8,9 Finally, elevated lipoprotein(a) [Lp(a)] levels are independently associated with increased cardiovascular risk due to the activation of atherogenic, inflammatory and prothrombotic mechanisms. 10
Considering the above, the aims of this study were:
1) to determine the prevalence of risk modulators [CAP, CAC score and Lp(a) level] in a primary pre vention population initially stratified through a risk score; 2) determine the concordance between the 2 methods of detecting subclinical atheromatosis (CAP and CAC score); 3) establish which proportion of patients should receive statins according to the initial risk stratification and after being recategorized by screening for risk modulators.
METHODS
This is a descriptive cross-sectional study with a sample obtained from the cardiovascular prevention outpatient clinic belonging to the Department of Cardiology of a private health center in the Autonomous City of Buenos Aires.
Patients between 18 and 79 years, without cardiovascular disease or previous lipid-lowering treatment who attended the clinic for cardiovascular risk assessment were included.
Definition of variables
The risk score (ASCVD Risk Estimator) used by the American guidelines for the management of cholesterol (American College of Cardiology/American Heart Association) was calculated in patients within the age range and with the variables required to calculate it. 11 Patients with a score < than 5%, between 5% and 7.4%, between 7.5% and 19.9%, and equal to or greater than 20% were classified as low risk, "borderline" risk, moderate risk, and high risk, respectively. The presence of CAP was non-invasively assessed by ultrasound and was defined as: 1) abnormal wall thickness (defined as intima-media thickness > 1.5 mm); 2) abnormal structure (protrusion towards the lumen, loss of alignment
with the adjacent wall); and 3) abnormal wall echogenicity. The CAC score was calculated using a multislice computed tomography without contrast agent injection and with electrocardiographic gating during a breath-hold of approximately 5-7 seconds. Coronary calcium was identified as an area of at least 1 mm2 at the level of the vessel with a threshold > 130 Hounsfied units. The CAC score obtained was expressed in Agatston units.
Lp(a) plasma level was obtained by the nephelometric method and was expressed in mg/dL. A value of 50 mg/dL or greater was considered elevated. Triglyceride levels, total cholesterol and HDL-cholesterol (HDL-C) were measured by enzymatic-colorimetric assay method and immunoturbidimetry. LDL-cholesterol (LDL-C) was calculated with the Friedewald formula. 12 Glomerular filtration rate was estimated using the CKD-Epidemiology Collaboration (CKD- EPI) equation. 13 All the tests were performed in a single laboratory with the same methods.
For this study, and based on current recommendations, statins were indicated for the following groups of patients in primary prevention, after the initial stratification by clinical data and risk score: a) severe hypercholesterolemia (C-LDL 190 mg/dL); b) diabetes mellitus; c) moderate or severe renal dysfunction (glomerular filtration rate < 60 mL/min/1.73 m2); d) high risk score. 11-15 With the information obtained about the presence of risk modulators, the following clinical scenarios were also considered for recommending statins according to different guidelines, position papers, and consensus statements: (a) patients with borderline or moderate risk with CAC score > 0 14; (b) low-risk patients with a CAC score 100 or greater than the expected 75th percentile for sex and age 11,15,16; c) patients with low, borderline or moderate risk with CAP 12,13; d) subjects with moderate risk and a Lp(a) value 50 mg/dL (17); and e) subjects with low or borderline risk and a Lp(a) value 75 mg/dL. 17
Statistical analysis
The normal distribution of the variables was explored by analyzing mean, standard deviation, median, skewness, kurtosis, a histogram and with the Shapiro-Wilk test. The difference of continuous variables between the groups were compared using the Student's t test or Mann-Withney-Wilcoxon test for normal and abnormal distributions, respectively. Categorical variables were analyzed with the chi-square test. The agreement between the two methods for quantifying subclinical atheromatosis (CAC by computed tomography and CAP by ultrasound) was analyzed using Fleiss' kappa. Agreement was defined as poor, fair, moderate, substantial, or almost perfect if the kappa value was less than or equal to 0.20, between 0.21 and 0.40, between 0.41 and 0.60, between 0.61 and 0.80, and between 0.81 and 1, respectively. Continuous variables were expressed as mean ± standard deviation or median (25-75 interquartile range), as applicable, and categorical variables as percentages. A twotailed p value < 0.05 was considered statistically significant. All the statistical calculations were performed using STATA 11.1 software package.
RESULTS
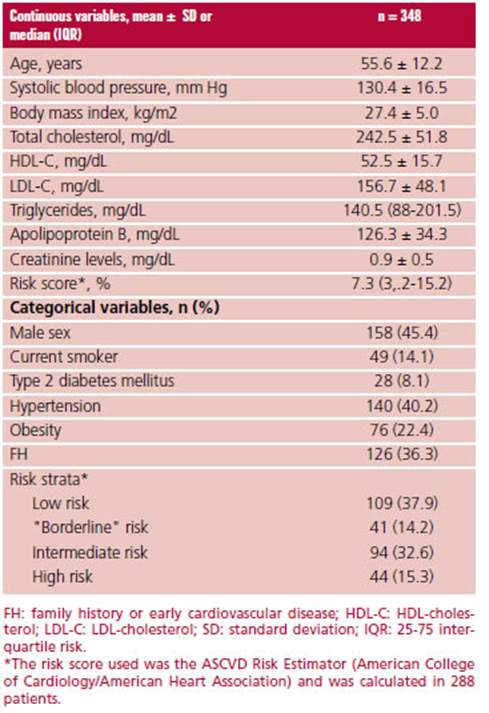
A total of 348 patients in primary prevention who were not receiving lipid-lowering treatment were included in our study. Mean age was 55.6 ± 12.2 years and 45.4%% were men; 8.1% had a history of type 2 diabetes mellitus and 40.2% had hypertension. Mean total cholesterol was 242.5 ± 51.9 mg/dL. The characteristics of the study population are described in Table 1.
The adequate characteristics to estimate the risk score were found in 82.8% (n = 288) of the population. When this subpopulation was analyzed, 37.9%, 14.2%, 32.6% and 15.3% of patients were classified at low, "borderline", moderate or high risk, respectively.
The risk score could not be estimated in 17.2% (n = 60) of the population due to age < 40 years (n = 37), presence of severe hypercholesterolemia, total cholesterol > 320 mg/dL, (n = 20) or both conditions (n = 3).
Overall, 29.8%, 36.8%, and 53.2% of patients showed a Lp(a) value 50 mg/dL, CAP, or a CAC score 0, respectively. A CAC score 100 or 300 was present in 17.5% and 8.3% of subjects, respectively, while only 5 patients had a score 1000.
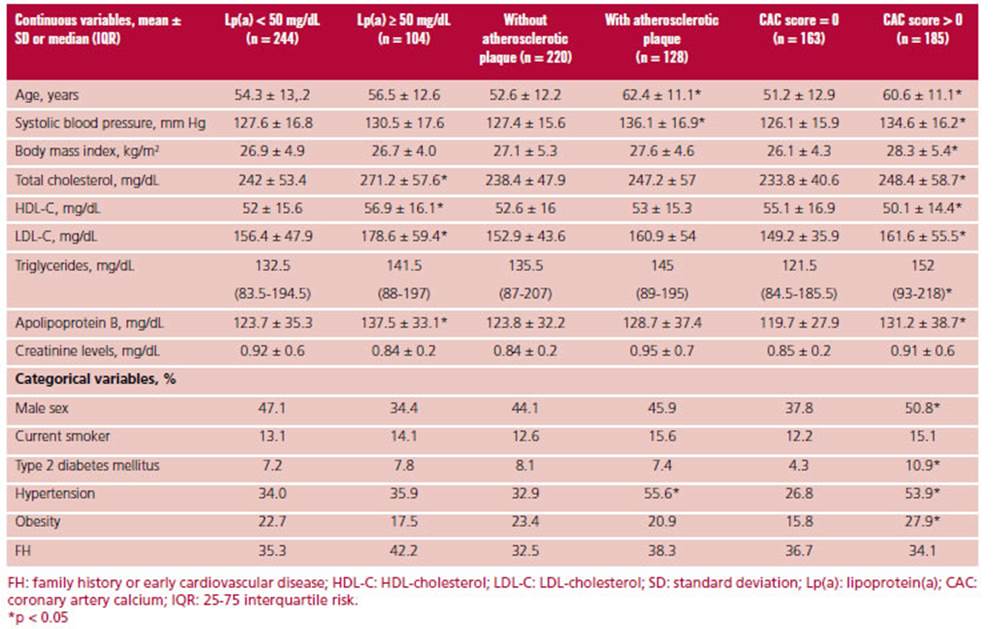
Patients with elevated Lp(a) levels showed higher total cholesterol, LDL-C, HDL-C and apolipoprotein B values compared with those with low levels. Patients with CAP were older and were more commonly hypertensive compared to patients without carotid atheromatosis. Finally, risk factors (including age, male sex, lipid values, obesity, diabetes, and hypertension) were more common in individuals with a CAC score > 0 compared with patients with a CAC score = 0. The characteristics of the population according to the presence or absence of risk modulators are shown in Table 2.
In the subpopulation stratified by risk score (n = 288), 27.2% had Lp(a) levels 50 mg/dL, with a median of 25 (9.9-57.7) mg/dL. Coronary calcium score was 0 in 55.9% of the subjects and 39.2% presented CAP. In addition, CAC score was 100 or the 75th percentile expected for sex and age in 18.9% and 29.6% of patients. The presence of risk modulators in the different categories estimated by the cardiovascular risk score can be observed in Figure 1. Despite the lack of indication for statins, a significant proportion of low- risk subjects (n = 109) had risk modulators: 25.7% had Lp(a) 50 mg/dL; 22% had CAP; 33% had CAC score > 0, and 33% had CAC score 75th percentile for sex and age.
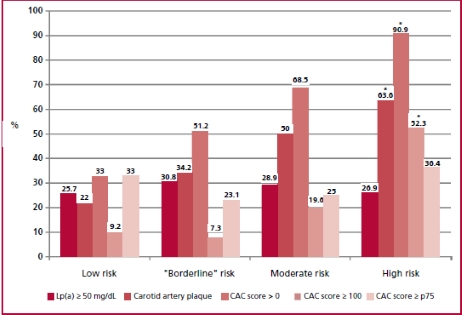
Fig 1. Prevalence of risk mod ulators in the different categories of cardiovascular risk factors (n = 288) *p < 0.05.
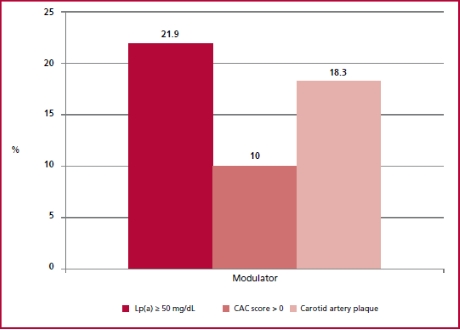
In the subpopulation that could not be stratified using the risk score due to age < 40 years and in those with total cholesterol < 320 mg/dL (n = 37), median Lp(a) was 18.6 (9.8-36.3) mg/dL, and was 50 mg/dL in 18.9% of patients. In 8.1% of patients CAC score was > 0 and 2.7% had CAP, with an overall prevalence of subclinical atherosclerosis of 11%. It is worth mentioning that in subjects < 45 years and with total cholesterol < 320 mg/dL (n = 60) the prevalence of CAP was 18.3% while 10% had CAC score > 0 (none of the patients had CAC score 100). The total prevalence of subclinical atheromatosis in subjects < 45 years was 23%. Figure 2 shows the graphical representation of the prevalence of cardiovascular risk modulators in this subpopulation.
In the subpopulation that could not be stratified using the risk score due to severe hypercholesterolemia (n = 23), the median Lp(a) was 54.3 (20-72) mg/dL and was 50 mg/dL in 55% of patients. In addition, 91.3% and 65.2% of patients had a CAC score > 0 or associated CAP, respectively. In addition, 25% and 66.7% of patients had CAC score 100 or 75th percentile expected for age. The graphical representation of the prevalence of cardiovascular risk modulators in this subpopulation can be observed in Figure 3.
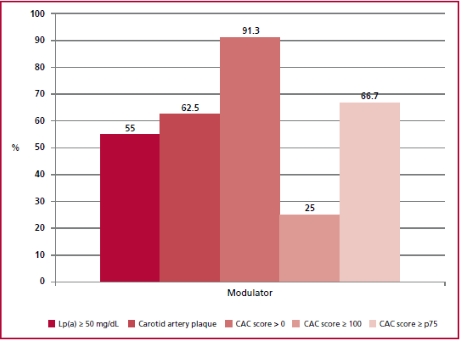
Fig. 3 Prevalence of risk modulators in the patients with severe hypercholesterolemia (>320 mg/dL, n = 23). Lp(a): Lipoprotein(a); p75: percentil 75; CAC: coronary artery calcium.
Of the total of patients with CAP, 75.8% had a CAC score > 0. Moreover, of all the patients with a CAC score > 0, 52.4% had CAP. The agreement between the two methods for quantifying subclinical atheromatosis was fair (kappa= 0.33), both in men (kappa = 0.35) and women (kappa = 0.30). In patients 40 years, agreement was fair (kappa = 0.29), and was moderate (kappa = 0.45) in younger patients.
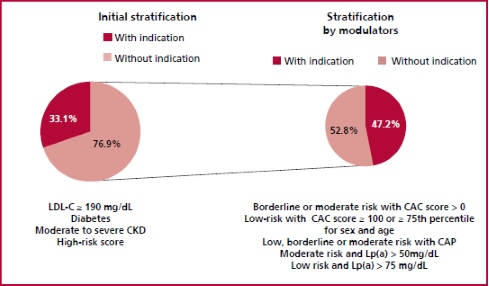
The initial stratification based on clinical data and risk score showed that 33% (n = 115) of the population would be candidates to receive statins. However, when the presence of risk modulators was considered, 110 patients (47.2%) who were initially not considered candidates for lipid-lowering agents had indication for statins (Figure 4).
DISCUSSION
Our study exposes the limitations of estimating cardiovascular risk based exclusively on a risk score, since many young patients or with low-risk score had subclinical atheromatosis or elevated lipoprotein(a) levels.
In our study, the risk score could not be assessed in 37 subjects < 40 years without severe hypercholesterolemia. In the 60 patients < 45 years despite the CAC score was available, we could not evaluate the percentile >75th by sex and age, since the epidemiological studies did not report the percentiles in this age group. These results are clinically relevant since approximately 3% to 10% of acute coronary syndromes occur in very young patients. 18 Despite these results, cardiovascular risk is often underestimated in this population. Very young patients with a first episode of coronary artery disease have high prevalence of overweight, smoking and hyperlipidemia. 19 Our data showed that approximately 1 out of 4 to 5 subjects < 45 years had subclinical atheromatosis (18% CAP, 10% CAC score > 0). Similar findings were recently reported in a study by Razavi et al. in which 1 out of 10 young people in the general population and one out of three young people with traditional risk factors had a high CAC score. 20 In addition, a cohort study including more than 13 000 subjects aged 30-49 years reported a prevalence of CAC score > 0 in 20.6%. At long-term follow-up, CAC score was an independent predictor of vascular events and mortality. 21 Also, another study evaluated the presence of subclinical atheromatosis in a very young population (between 14 and 40 years old). The prevalence of CAP was 5.2%, and even more relevant, its presence was independently associated with a higher incidence of cardiovascular events and mortality during follow-up. 22
On the other hand, although our study showed that the higher risk estimated with the risk score was associated with higher prevalence of subclinical atheromatosis, one third of the patients categorized as "low risk" had CAC score > 0 and 1 out of 5 subjects had CAP. Similar results were reported in previous studies, in which 20-30% of patients categorized as "low risk" by different scores exhibited CAP. 23,24 Likewise, in large population-based studies, 11% to 15% "low-risk" patients were recategorized when CAC score was considered. 25 Although the percentages are lower than those reported in our paper, our sample of patients is made up of individuals who consulted for cardiovascular risk assessment and had a higher prevalence of risk factors compared to the general population.
Subjects with severe hypercholesterolemia (many of them with familial hypercholesterolemia), who could not be stratified using the risk score constitute a population at increased cardiovascular risk. Although the presence of risk modulators in this population adds prognostic information, 26-29 it does not change the initial indication for high-intensity statins. Our study showed a high prevalence of risk modulators in this subpopulation, in agreement with other publications. Only less than 9% of patients with severe hypercholesterolemia had no subclinical coronary atheromatosis, whereas 65% had atherosclerotic involvement of both territories.
The current evidence suggests that estimation of CAC score better predicts cardiovascular events than the presence of CAP. 30 However, the agreement between both determinations to detect subclinical atheromatosis was low in our study. In other words, and consistent with the data we have previously reported, a "normal" carotid Doppler ultrasound does not exclude the presence of subclinical coronary artery atherosclerosis, and vice versa. 31 Furthermore, our findings coincide with those reported by Moreyra et al. in that the agreement between the coronary and carotid territories in subjects undergoing primary prevention was weak (kappa 0.21). 32
Another interesting finding of our paper is that approximately 25-30% of patients showed an elevated Lp(a) level, including the low-risk subgroup. Except for lipid profile, there was no significant association between high Lp(a) values and traditional risk factors. The association between elevated Lp(a) values and increased risk of cardiovascular disease has emerged from epidemiological and genetic studies. 33,34 The activation of pro-atherogenic, pro-inflammatory and pro-thrombotic mechanisms would explain the association of this lipoprotein with increased cardiovascular risk. 35 Recently, a European position paper recommends measuring Lp(a) concentration at least once in a lifetime in the adult population. Without specific therapies to reduce Lp(a) levels, this consensus recommends counteracting elevated Lp(a) by early and intensive control of risk factors, mainly by treating hypertension and reducing LDL-C. 17
In addition, several guidelines on cardiovascular prevention recommend screening for subclinical atheromatosis, mainly in "intermediate risk" patients, as a useful means of recategorizing them. 11,14,16 The main aim of recategorization is to better identify patients who will benefit from the use of statins. Lipid guidelines, recommendations, and consensus statements also use the detection of risk modulators to rec ommend C-LDL targets and the intensity of lipid-lowering therapy. 11-16 Our work showed that 47.2% of the subjects who were not initially candidates for receiving statins, were so when they were reclassified through the detection of risk modulators. Therefore, using this hybrid modality of vascular risk assessment, we would recommend the indication of statins in 65% of our population sample. In our opinion, and considering our results, screening for risk modulators should also be considered in low-risk strata.
Finally, the strategy of estimating cardiovascular risk based on risk scores was practically not evaluated in randomized clinical trials. Recently, a study showed that risk stratification based on CAC score compared with a strategy based on risk score may be more efficient, personalized, cost-effective, and motivating for statin initiation and maintenance in patients in primary prevention. 36 Whether this further translates into a reduction in cardiovascular events is being evaluated in a large, long-term trial. 37
Our study has certain limitations. Firstly, in we only used the risk score recommended by the American guidelines. The implementation of other risk functions could change the results. Secondly, the criteria used to indicate statins were selected by the group of researchers, based on current local and international guidelines. Finally, the presence of biases and confounders may be expected due to the observational design of our study.
CONCLUSION
The presence of risk modulators was common in this population in primary prevention, even in low-risk subjects or young individuals. Considering simultaneous screening of several risk modulators could optimize the initial stratification of our patients and lead to reconsider treatment with statins.