Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO  uBio
uBio
Compartir
Boletín de la Sociedad Argentina de Botánica
versión On-line ISSN 1851-2372
Bol. Soc. Argent. Bot. vol.57 no.4 Córdoba dic. 2022
http://dx.doi.org/10.31055/1851.2372.v57.n4.35662
10.31055/1851.2372.v57.n4.35662
Palinología
Pollen and spores morphology from the Holocene of the Iberá Wetlands in northeastern Argentina
Morfología del polen y esporas del Holoceno de los Esteros del Iberá en el noreste de Argentina
LionelFernandez Pacella
MercedesDi Pasquo
1. Centro de Ecología Aplicada del Litoral (CECOAL-CONICET-UNNE), Dpto. Biología-FaCENA-UNNE, Ruta 5, Km 2,5, Corrientes, Argentina.
2. Laboratorio de Palinoestratigrafía y Paleobotánica, CICyTTP-CONICET, Dr. Materi y España s.n., Diamante, Entre Ríos, Argentina. medipa@ cicyttp.org.ar
*lionelpacella@yahoo.com.ar
Summary
Background and aims: Taxonomic works of the modern pollen grains from the vegetation of the Iberá Wetlands from northeastern Argentina, and other areas of the Corrientes province, have mostly been carried out in last decade. However, very few of these taxonomic works include illustration of palynomorphs. The objective of this contribution is to provide the first morphological records of palynomorphs of angiosperms, ferns, lycophytes and bryophytes from sediments from the Holocene of the Iberá Wetlands.
M&M: Core samples obtained by mean of a Livingston-type sampler from six lakes in their central and deepest parts on the western margin of the Iberá Wetlands were analyzed.
Results: Fifty-five types of palynomorphs are described and illustrated: 46 pollen types correspond to 27 families of angiosperms and nine trilete spore-types to ferns, lycophytes and bryophytes. Information to differentiate local (species that form part of the natural vegetation of the Iberá Wetlands) and extra-local taxa (those that do not belong to the Iberá), that achieved to depocenters mostly by means of wind currents coming from farther regional vegetation, was included.
Conclusions: Pollen grains and spores identifications up to species level enhances paleoenvironmental reconstructions based on more accurate ecologic information and geographical distribution. This work broadens the knowledge of the palynological flora of northeastern Argentina and it will contribute to differentiate the local vegetation from the extra-local in future paleocological and paleoenvironmental interpretations.
Keywords: Corrientes Province, hydrographic system Iberá, holocene, palynomoph.
Resumen
Introducción y objetivos: Los trabajos taxonómicos de polen actual de la vegetación de los esteros del Iberá en el noreste de Argentina y otras áreas de la provincia de Corrientes se han llevaron a cabo mayoritariamente en la última década. Sin embargo, muy pocos de estos trabajos incluyen la ilustración de los palinomorfos. El objetivo de esta contribución es proporcionar el primer registro morfológico de palinomorfos de angiospermas, helechos, licofitas y briofitas de sedimentos del Holoceno de los Esteros del Iberá.
M&M: Se analizaron muestras de núcleos obtenidos por medio de un muestreador tipo Livingston de seis lagos en sus partes central y más profunda en el margen occidental de los Esteros del Iberá.
Resultados: Se describen e ilustran 55 tipos de palinomorfos: 46 tipos de polen corresponden a 27 familias de angiospermas y nueve tipos de esporas triletas a helechos, licofitas y briofitas. Se incluye información para diferenciar taxones locales (que forman parte de la vegetación natural de los Esteros del Iberá) y extralocales (aquellos que no pertenecen al Iberá) que lograron llegar a los depocentros provenientes de vegetación regional más lejana.
Conclusiones: La identificación de granos de polen y esporas hasta el nivel de especie mejora las reconstrucciones paleoambientales basadas sobre información ecológica y distribución geográfica más precisas. Este trabajo amplía el conocimiento de la flora palinológica del noreste de Argentina y contribuirá a diferenciar la vegetación local de la extra-local en futuras interpretaciones paleoecológicas y paleoambientales.
Palabras clave: Holoceno, palinomorfo, Provincia de Corrientes, sistema hidrográfico Iberá.
Introduction
The geological history of the región currently known as Iberá Wetlands in central-eastern South America, currently northeastern Argentina, begins to have its own identity from the tectonic movements that determined the rise of the Andean mountain range (Ramos, 1999). Orogeny unleashed compressive forces from the East that fractured the powerful basaltic lava flows belonging to the Solari-Serra Geral Formation (Cretaceous) accumulated in the study region (Herbst & Santa Cruz, 1985). Thus, large blocks of rock generated and separated by several fault systems with dominant NE-SW and NW-SE strikes. The subsequent epirogenic movements gave rise to the independent adjustment of the megablocks, modeling the topography of the subsoil covered by sedimentary fill (Gentili & Rimoldi, 1979). This generated in northeastern Argentina, province of Corrientes, the extensive depression that crosses it almost entirely in a NE-SW direction, on which important fluvial runoff routes were developed. At the end of the Pliocene, water excesses were concentrated in these depressed lands, initiating its geological activity in the current Paraná River. Later tectonic movements accentuated the vertical displacement of the subsoil blocks, triggering changes in the runoff design of surface waters, and being a consequence in the province of Corrientes, the Paraná River migrated from South to North until it occupied its current position, which evidences a clear structural control (Iriondo, 1994). The central depression of the Corrientes territory disconnected from the Paraná River, in the surrounding of the town of Ituzaingó, where the old river valley transformed into a shallow basin, was inefficient to evacuate excess of water. This gave rise to the development of a complex association of lentic environments fed fundamentally by precipitation and probably by the Paraná River due to subterranean transfluence (Orfeo, 2005).
Therefore a complex association of lentic and lotic environments distributed over large areas of the Iberá Wetlands in eastern Corrientes developed during the Quaternary (Neiff, 1997; Iriondo, 2010), from which taxonomic works of modern pollen grains of floristic biomes carried out mostly in the last decades (e.g. Fernandez Pacella, 2013, 2014). This botanical and ecological information is relevant to construct modern analogs of this region applied in palynological studies of surface and subsurface samples, although very few taxonomic works with illustration of palynomorphs published up to today (Anzótegui & Garralla, 1985). Cuadrado & Neiff (1993) contributed with the first analysis of disperse pollen from samples collected in dammed vegetation ("embalsados") located in eastern region of Iberá Wetlands. These authors established that only upper levels yielded pollen grains representative of the current vegetation. They also found that pollen grains are poorly preserved, even physic and chemical features (e.g. low oxygen, low light, acid pH and high amounts of organic matter) could have favored the preservation of organic matter. Later, Garralla (1998) interpreted that the region was vegetated by xero-halophyte forests at around 3,500 years BP.
Characteristics of the study area
Iberá Wetlands is one of the most important tropical wetlands of the biosphere, in terms of its extension and diversity of both animals and plants. The toponymy referred to "waters that shine" (Y verá in Guarani language). It comprises a complex water system composed of, marshes, shallow lakes and interconnected river courses (Orfeo & Neiff, 2008).
The lagoons correspond to extensive and shallow plains fed mainly by the rains (1,200 to 1,500 mm year) and they are one of the most important wetlands in Latin America due to its extension with more than 12,000 km2. It is located in the central part of Corrientes with a major axis NE-SW direction (Fig. 1), where a complex association of lentic and lotic environments are vaguely and transitionally delineated (Neiff, 1997). Its extension into the Paraguay Republic "Esteros de Ñeembucú", bearing about 45,000 km2, are developed in its western area and confirms the great influence of the rivers in the maintainance of its fluvial plains such as the large Paraná (Neiff, 2004).
The plant communities of the study area in the Iberá Wetlands are included in the eastern district of the Chaco province. Its humid sandy plains are composed of typical savanna ofAndropogon lateralis Nees (Poaceae), accompanied by Cyperaceae and other species of Poaceae, developed in more or less elevated sectors well-drained; in depressions with slow permeability, this vegetation is sometimes transformed into marshes (Carnevalli, 1994). Deeper sand arcas ("espartillares"), that are well-drained soils of pluvial in origin, are colonized by Elionurus muticus (Spreng.) Kuntze (Poaceae), as well as grasslands of Sorghastrum agrostoides (Speg.) Hitchc., Paspalum sp. and Spartina sp. (Poaceae) generally located in floodable soils like streams and marshes. The hygrophilous forest (e.g. Poaceae, Leguminosae) extended over wavy red sands (Carnevalli, 1994).
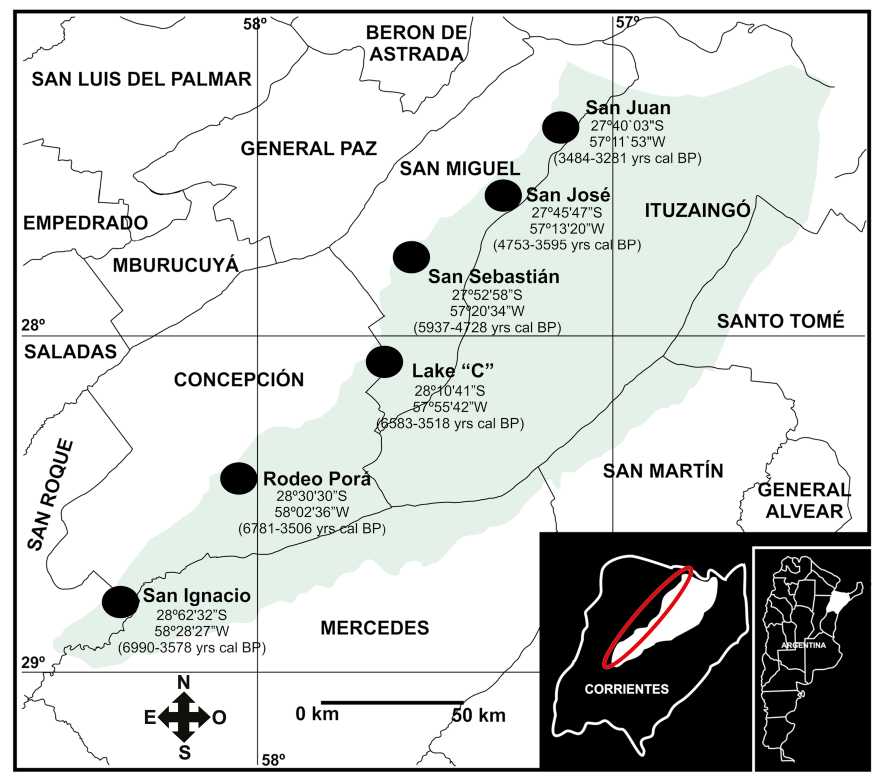
Fig. 1. Map of the western margin of the Iberá Wetland showing the sequence of the lakes studied, and cal BP age range for each lake (from Fernandez Pacella & Lara, 2019).
Paleoenvironment during the Holocene in the study area
Palynomorphs were identified in lacustrine sediments of northwestern of the Iberá Wetlands (Fernandez Pacella et al., 2011, 2013; Fernandez Pacella & Lara, 2019) which represented mostly marsh-herbaceous vegetation under humid condition between 5968-5000 years cal BP. In agreement with Iriondo (1994), drier conditions were determined from 5000 to 3506 years cal BP with a decrease in the species frequency typical of wet environments and increase of xero-halophyte vegetation. This author pointed out that the influence of template climate in Patagonia would have affected the southern region of Corrientes evidencing changes in landscapes. The clogging of the water body occurred from 3484 years cal BP onwards when herbaceous vegetation dominated.
The appearance of arboreal pollen indicates the beginning of the modern hygrophilous forest. Those paleoenvironmental changes of alternating wetter and drier episodes recorded in this region are supported by fields of eolian dunes in Iberá Wetlands, developed during the drier periods in the late Holocene (Iriondo, 2010).
The objective of this contribution is to provide the first morphological documentation of angiosperms, ferns, lycophytes and bryophytes from core sediments of six lakes of the Holocene western margin of the Iberá Wetlands. Species from local versus extra-local vegetation differentiated in the taxonomic description in order to contribute to a better understanding of the floral composition of the Iberá Wetlands and environmental changes yet documented during the Holocene.
Materials and Methods
The taxonomic analysis of pollen grains and spores presented in this contribution is a new study derived from previous studies carried out by Fernandez Pacella et al. (2011), Fernandez Pacella (2013) and Fernandez Pacella & Lara (2019) in which paleoenvironmental interpretations based on main palynologic groups addressed. These works included the study of core samples obtained with a Livingston-type sampler from six lakes in their central and deepest parts on the western margin of the Iberá Wetlands. The interval studied of each core dated by C14 spans c. 6000 to 3000 Years BP (Fig. 1). Samples were taken each 5 cm from five lakes (San Juan, 75 cm, 27° 40' 0" S 57° 11' 53" W; San José, 85 cm, 27° 45' 47" S 57° 13' 20" W; Lake "C", 110 cm, 28° 10' 41'' S 57° 55' 42'' W; Rodeo Porá, 120 cm, 28° 30' 30'' S 58° 2' 3'' W; San Ignacio, 145 cm, 28° 32' 32'' S 58° 28' 27'' W). From the San Sebastián lake (24 cm, 27° 52' 58'' S 57° 20' 34'' W) the interval of sampling used was each 2 cm.
The palynological maceration followed usual techniques of Faegri & Iversen (1989), which consisted on deflocculation of clays with (NaPO3)6 10%, elimination of humic acids with NaOH 5%, elimination of carbonates with HCl 10%, separation of organic matter from inorganic material with heavy liquids (ZnCl2) and elimination of silicates with HF.
The material processed and housed in the laboratory at the CECOAL (Centro de Ecología Aplicada del Litoral) of CONICET-UNNE (Universidad Nacional del Nordeste), in the collection "Dr. Rafael Herbst".
The morphological description was based on 30-50 pollen grains for each taxon, and it was done under optical microscope NIKON ECLIPSE E100 illustrated with a camera Nikon 590CU (40x and 100x magnification). We followed the classification of Curtis et al. (2001) and Raven et al. (1991) for pollen grains, and genera included in the APG VI Classification System (2017) and PPG I (2016). Terminology used for descriptions of pollen grains and spores is based on Kremp (1965), Nilson & Praglowski (1992), Punt et al. (2007), and Sáenz Laín (2004). The reference collection PAL-CTES of the UNNE and specialized literature were used to identify pollen grains and spores (Acevedo & Anzótegui, 1998; Anzótegui & Ferrucci, 1998; Anzótegui, 2001; Anzótegui & Caccavari, 2001; Anzótegui & Mautino, 2001a, b; Bhattacharya et al., 2009; Caccavari & Dome, 2001, 2006; Cuadrado, 1998a, b, c; Fernandez Pacella & Canteros, 2014; Fernandez Pacella et al., 2014a, b; Fuertes & Rodríguez, 2009; Garralla & Cuadrado, 2001; Markgraf & D'Antoni, 1978; Pire et al., 2006).
Local taxa refer to those species that are part of the natural vegetation of the Iberá Wetlands (Arbo & Tressens, 2002) whereas the extra-local taxa are those that do not belong to the Iberá. The latter species found in low percentages in the Tilia diagrams of the lakes analyzed by Fernandez Pacella et al. (2011) and Fernandez Pacella & Lara (2019). They achieved to depocenters by means of wind currents coming from farther regional vegetation of N, NW and W of Corrientes and E of Chaco (Carnevali, 1994; Fontana, 2018). This is due to the Iberá Wetlands was probably a closed basin made up of an extensive mosaic of lentic bodies fed by rain and without connection with rivers or streams during the Mid-Late Holocene (Neiff, 2004; Orfeo, 2005).
Results
Fifty-five palynomorphs were recorded in the study area of the Iberá Wetlands. Forty-six of them are pollen types corresponding to 27 families of angiosperms and nine trilete spore-types of ferns, lycophytes, and bryophytes that are described and illustrated (Figs. 2-5). In the taxonomic description, helpful information to differentiate local and extralocal taxa for each site studied is included (Table 1).
The 46 recorded species with pollen are listed alphabetically by family:
Acanthaceae (Magnoliopsida, Lamíales)
Justicia brasiliana Roth, Nov. Pl. Sp.: 17. 1821. Fig. 2A-C.
Morphology: Diporate pollen grain, radial symmetry. Circular amb, (PA) 17-84 pm, prolate (EA) 14-43 pm. Lalongate endoapertures, 2-7 pm diam. Exine 1-5 pm thick, thinner in apertural area, 0.5-1 pm thick, sexine semitectate, reticulated, heterobrocate reticulum, with straight or sinuous walls and circular or polygonal lumens. Surrounding the aperture differentiate and contain 2-6 rows of 4-10 islands (Pire et al., 2006).
Ecological procedence: grass or shrub well represented in forests of S. balansae and M. balansae, corresponding to extra-local taxa of Corrientes (Fontana, 2018). Native species of Chacoan and Paranaense floristic provinces (Anton & Zuloaga, 2021).
Amaranthaceae (Magnoliopsida, Caryophyllales)
Amaranthus muricatus (Moq.) Hieron., Bol. Acad. Nac. Ci. 4: 421. 1882. Fig. 2D.
Morphology: Pantoporate pollen grain, radial symmetry, spheroidal, small size, diameter 1922 pm. Apolar. Circular pores, 28-45, with thick margin, distributed all over the grain surface, 1-2 pm in diameter. Exine 1.2 a 1.5 pm thick. Microreticulate (Cuadrado, 1998a).
Ecological procedence: herbaceous species frequent in natural grasslands of the Iberá Wetlands (Arbo & Tressens, 2002) and mainly in North-central Argentina (Anton & Zuloaga, 2021).
Gomphrena perennis L., Sp. Pl. 1: 224. 1753. Fig. 2E, F.
Morphology: Pantoporate pollen grain, radial symmetry. Spheroidal, 14-22 pm. Apolar. Pores 30-37, 1.2-2.3 pm diam. Exine 1.5-3.5 pm thick, metareticulate, with hexagonal elements (Cuadrado, 1998a).
Ecological procedence: herbaceous species in grasslands of the Iberá Wetlands (Arbo & Tressens, 2002), and North-central region of Argentina (Anton & Zuloaga, 2021).
Amaryllidaceae (Liliopsida, Asparagales)
Crinum americanum L., Sp. Pl. 1: 292. 1753. Fig. 2G.
Morphology: Monosulcate pollen grain, radial symmetry. Small size, suboblate, (PA) 12-15 pm, (EA) 16-18 pm. Heteropolar. Sulcus 2 x 4 pm. Exine 2 pm thick, sexine tectate, microechinate of ca. 1 pm (Medeanic et al., 2008).
Ecological procedence: herbaceous local species well represented in marshes of the natural vegetation of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002).
Anacardiaceae (Magnoliopsida, Sapindales)
Myracrodruon balansae (Engl.) Santin, Revista Brasil. Bot. 14: 135. 1991. Fig. 2H, I.
Morphology: Tricolporate pollen grain, radial symmetry. Subtriangular amb, subprolate, (PA) 21-28 pm, (EA) 16-24 pm. Isopolar. Colpi 2.5 pm wide, ribs 1.4-2 pm thick, lalongate endoapertures 1.4-2.1 x 7-10 pm. Exine 1.4 pm thick. Sexine semitectate. Surface striate, short and wide striations lesser than 2.5 pm in length, and larger than 1.7 pm in width, walls 0.5 pm wide and 0.2-0.3 pm high (Anzótegui, 2001).
Ecological procedence: native extra-local tree represented in forests of S. balansae and M. balansae, of the natural vegetation of Corrientes (Boelcke, 1992; Fontana, 2018) and other region of Chacoan and Paranaense floristic provinces (Anton & Zuloaga, 2021).
Schinopsis balansae Engl., Bot. Jahrb. Syst. 6: 286. 1885. Fig. 2J.
Morphology: Tricolporate pollen grain, radial symmetry. Prolate, (PA) 32-35 pm, (EA) 24-27 pm. Isopolar. Colpi 1-2.5 pm maximum equatorial in width, acute or blunt apices, ribs 1.4-3.5 pm thick at the equator. Lalongate endoapertures 3-4 x 7-8 pm. Exine 0.7-2 pm thick. Surface striate, long striations, longer than 2.5 pm and 1 pm or less in width, walls slightly taller than 0.5 pm in height (Anzótegui, 2001).
Ecological procedence: native extra-local species of the natural vegetation of Corrientes in forests of S. balansae and M. balansae (Fontana, 2018) belonging to the Chacoan floristic province (Anton & Zuloaga, 2021).
Schinus longifolia (Lindl.) Speg., Cat. Descr. Maderas: 413. 1910. Fig. 2K.
Morphology: Tricolporate pollen grain, radial symmetry. Prolate, (PA) 18-21 pm, (EA) 14-16 pm. Isopolar. Colpi 1-2.5 pm wide, with 1.4-3.5 pm thick ribs at the equator limited by a small psilate margin of 2 pm wide. Endoapertures 1.4 x 4 pm in size. Exine 0.7-2.5 pm thick. Surface striate, long striations, approximately more than 2.5 pm long (Anzótegui, 2001).

Fig. 2. Pollen of Justicia, Amaranthus, Gomphrena, Crinum, Myracrodruon, Schinopsis, Schinus, Syagrus, Alnus, Tecoma, Celtis, Anthemis, Baccharis, Bidens, Conyza, and Gaillardia. A: Justicia brasiliana, equatorial view in superior focus (100x). B: Equatorial view in optical section (100x). C: Equatorial view in optical section (100x). D: Amaranthus muricatus, general view in superior focus where endoapertures are appreciated (100x). E: Gomphrena perennis, general view in optical section (100x). F: General view in superior focus (100x). G: Crinum americanum, polar view in optical section (100x). H: Myracrodruon balansae, equatorial view in superior focus (100x). I: Equatorial view in optical section (100x). J: Schinopsis balansae, equatorial view in optical section (100x). K: Schinus longifolia, equatorial view in optical section (100x). L: Syagrus romanzoffiana, polar view in optical section (100x). M: Alnus acuminata, polar view in optical section (100x). N: Tecoma stans, polar view in optical section (100x). O: Celtis iguanaea, equatorial view (100x). P: Anthemis cotula, polar view in superior focus (100x). Q: Baccharis trimera, polar view in optical section (100x). R: Bidens subalternans, polar view (100x). S: Conyza pampeana, polar view in superior focus (100x). T: Gaillardia megapotamica, equatorial view (100x). Scales= 10 pm.
Table 1. Pollen and spores procedence for each site studied. Abreviatures= L: local taxa; EL: extra-local taxa.
| Species or genus | Procedence | San Ignacio | Rodeo Porá | Lake "C" | San Sebastián | San José | San Juan |
| Alnus acuminata | L | X | X | ||||
| Amaranthus muricatus | L | X | X | X | X | ||
| Anadenanthera colubrina | EL | X | X | X | |||
| Anthemis cotula | L | X | X | X | X | ||
| Anthoceros aff. lamellatus | L | X | X | X | |||
| Baccharis trimera | L | X | X | X | X | X | |
| Bidens subalternans | L | X | X | X | X | X | X |
| Calliandra parvifolia | EL | X | X | ||||
| Celtis iguanaea | L | X | X | X | X | X | |
| Chrysophyllum marginatum | L | X | X | X | X | ||
| Cissus verticillata | L | X | X | X | |||
| Conyza pampeana | L | X | X | X | X | ||
| Crinum americanum | L | X | X | X | |||
| Cyathea aff. atrovirens | L | X | X | X | |||
| Cypella herbertii | L | X | X | X | X | ||
| Cyperus rotundus | L | X | X | X | X | X | X |
| Echinochloa crus-galli | L | X | X | X | X | X | X |
| Echinochloa polystachya | L | X | X | X | X | X | X |
| Empetrum aff. rubrum | L | X | X | X | |||
| Eryngium elegans | L | X | X | X | X | X | X |
| Gaillardia megapotamica | L | X | X | X | X | X | |
| Gomphrena perennis | L | X | X | ||||
| Gutierrezia resinosa | L | X | X | X | X | ||
| Hymenachne pernambucensis | L | X | X | X | X | X | X |
| Imperata brasiliensis | L | X | X | X | X | X | X |
| Jamesonia flexuosa | L | X | X | ||||
| Justicia brasiliana | EL | X | X | X | X | ||
| Ludwigia peploides | L | X | X | X | |||
| Mikania cordifolia | L | X | X | X | X | ||
| Myracrodruon balansae | EL | X | X | X | |||
| Myrcianthes pungens | EL | X | X | X | |||
| Myriophyllum aquaticum | L | X | X | ||||
| Pellaea sp. | L | X | X | ||||
| Phaeoceros bulbiculosus | L | X | X | ||||
| Phaeoceros tenuis | L | X | X | ||||
| Phlegmariurus aff. mandiocanus | L | X | X | ||||
| Phyllostylon rhamnoides | EL | X | X | X | |||
| Pisonia aculeata | EL | X | X | X |
| Species or genus | Procedence | San Ignacio | Rodeo Porá | Lake "C" | San Sebastián | San José | San Juan |
| Plinia rivularis | L | X | X | X | X | ||
| Polygala leptocaulis | L | X | X | ||||
| Polygonum acuminatum | L | X | X | X | |||
| Polygonum convolvulus | L | X | X | X | |||
| Prosopis alba | EL | X | X | X | |||
| Schinopsis balansae | EL | X | X | X | X | ||
| Schinus longifolia | EL | X | X | X | |||
| Selaginella aff. marginata | L | X | X | X | X | ||
| Senecio bonariensis | L | X | X | X | X | X | |
| Senegalia bonariensis | L | X | X | ||||
| Serjania perulacea | EL | X | X | X | X | ||
| Sphagnum sp. | L | X | X | ||||
| Syagrus romanzoffiana | EL | X | |||||
| Tecoma stans | L | X | X | X | |||
| Thinouia mucronata | L | X | X | X | |||
| Trichilia elegans | EL | X | X | X | |||
| Typha domingensis | L | X | X | X | X |
Ecological procedence: extra-local shrub or tree well represented in the natural palm grove vegetation of Corrientes (Fontana, 2018) and in Chacoan and Paranaense floral provinces (Anton & Zuloaga, 2021).
Arecaceae (Liliopsida, Arecales)
Syagrus romanzoffiana (Cham.) Glassman, Fieldiana, Bot. 31: 382. 1968. Fig. 2L.
Morphology: Monosulcate pollen grain, bilateral symmetry. Medium size, oblate, (PA) 15-17 pm, (EA) 37-39 pm. Heteropolar. Sulcus 35.4 pm long. The exine is 1.6 pm thick, sexine tectate and psilate (Trigo & Fernández, 1995; Bauermann etal, 2010).
Ecological procedence: extra-local arboreal species well represented in small palm stocks and riparian natural vegetation in Corrientes (Boelcke, 1992; Carnevalli, 1994; Cabral & Castro, 2007).
Mainly endemic of Pampean and Paranaense floral provinces (Anton & Zuloaga, 2021).
Betulaceae (Magnoliopsida, Fagales)
Alnus acuminata Kunth, Nov. Gen. Sp. (quarto ed.) 2: 20. 1817. Fig. 2M.
Morphology: Pentazonoporate pollen grain of radial symmetry. Medium size, pentagonal amb (EA) 40-43 pm. Isopolar. Pores 3-5 pm, protrudent and vestibular. Exine 2 pm thick, psilate (Markgraf & D'Antoni, 1978).
Ecological procedence: extra-local tree represented in forests of S. balansae and M. balansae of Corrientes (Boelcke, 1992; Fontana, 2018).
Bignoniaceae (Magnoliopsida, Lamiales)
Tecoma stans (L.) Juss. ex Kunth, Nov. Gen. Sp. (quarto ed.) 3: 144. 1819. Fig. 2N.
Morphology: Brevicolpate pollen grain, radial symmetry. Subtriangular amb, (EA) 48-52 pm. Isopolar. Exine 3 pm thick, sexine semitectate. Microreticulate (Markgraf & D'Antoni, 1978).
Ecological procedence: shrub or small tree represented in the natural vegetation of the Iberá
Wetlands in hygrophilous forests (Fontana, 2018). Native of North-central region of Argentina (Anton & Zuloaga, 2021).
Celtidaceae (Magnoliopsida, Rosales)
Celtis iguanaea (Jacq.) Sarg., Silva 7: 64. 1895. Fig. 2O.
Morphology: Triporate pollen grain, radial symmetry. Subtriangular to circular amb, suboblate, (PA) 9-30 pm, (EA) 11-32 pm. Isopolar. Pores slightly sunken, circular 1-2 pm in diam., limited by a ring 0.5-2 pm thick. Exine 0.5-1.4 pm thick. Sexine tectate. Scabrate (Anzótegui & Mautino, 2001a).
Ecological procedence: shrub (creep) or tree represented in hygrophilous forests of the Iberá Wetlands and extra-local forests of S. balansae and M. balansae in Corrientes (Arbo & Tressens, 2002; Fontana, 2018). Native of central-northern region of Argentina (Anton & Zuloaga, 2021).
Compositae (Magnoliopsida, Asterales)
Anthemis cotula L., Sp. Pl. 2: 894. 1753. Fig. 2P.
Morphology: Tricolporate pollen grains of radial symmetry. Spheroidal, (PA) 23-24 pm, (EA) 22-23 pm. Isopolar. Exine 4.5 pm thick. Sexine tectate, echinate, spines 4 to 5 pm (Alonso, 2014).
Ecological procedence: herbaceous local taxon in grasslands of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002) in Corrientes and widely present in southern South America (Anton & Zuloaga, 2021).
Baccharis trímera (Less.) DC., Prodr. 5: 425. 1836. Fig. 2Q.
Morphology: Tricolporate, radial symmetry. Spheroidal, (PA) 15-16 pm, (EA) 14-15 pm. Isopolar. Exine 4 pm thick. Sexine tectate, echinate, spines 2-2.5 pm long (Markgraf & D'Antoni, 1978).
Ecological procedence: this genus is well represented in natural grassland vegetation of the Iberá Wetlands (Arbo & Tressens, 2002) and in North-central region of Argentina (Anton & Zuloaga, 2021).
Bidens subalternans DC., Prodr. 5: 600. 1836. Fig. 2R.
Morphology: Tricolporate pollen grain, radial symmetry. Oblate-spheroidal, (PA) 26-28 pm, (EA) 27-28 pm. Isopolar. Exine 2.5-3 pm thick, sexine tectate, echinate, spines 7-7.5 pm (Markgraf & D'Antoni, 1978).
Ecological procedence: herbaceous taxon presents in grasslands of the Iberá Wetlands (Arbo & Tressens, 2002).
Conyza pampeana (Parodi) Cabrera, Man. Fl. Al. Buenos Aires: 481. 1953. Fig. 2S.
Morphology: Tricolporate pollen grain of radial symmetry. Prolate to subcircular, (PA) 25-27 pm, (EA) 21-24 pm. Isopolar. Exine 4 pm thick. Sexine tectate, echinate, spines 2 pm (Medeanic et al., 2008).
Ecological procedence: this genus is well represented in the natural vegetation of the Iberá Wetlands in grasslands (Arbo & Tressens, 2002).
Gaillardia megapotamica (Spreng.) Baker, Fl. Bras. 6: 276. 1884. Fig. 2T.
Morphology: Tricolporate pollen grains of radial symmetry. Medium, suboblate, (PA) 27-30 pm, (EA) 34-37 pm. Isopolar. Exine 2 pm thick, sexine tectate, echinate, with spines 4 pm long (Markgraf & D'Antoni, 1978).
Ecological procedence: perennial grass or subshrub well represented in grasslands of the Iberá Wetlands (Arbo & Tressens, 2002).
Gutierrezia resinosa (Hook. & Arn.) S. F. Blake, Contr. U.S. Natl. Herb. 26: 232. 1930. Fig. 3A.
Morphology: Tricolporate pollen grain, radial symmetry. Small size, spheroidal, (PA) 14-18 pm, (EA) 14-18 pm. Isopolar. Exine 2 pm thick. Sexine tectate, echinate, spines 1.5 pm long (Markgraf & D'Antoni, 1978).
Ecological procedence: Gutierrezia Lag. is present in natural grasslands of the Iberá Wetlands (Arbo & Tressens, 2002).
Mikania cordifolia (L. f.) Willd., Sp. Pl. 3: 1746. 1803. Fig. 3B.
Morphology: Tricolporate pollen grains of radial symmetry. Medium size, subprolate (PA) 27-30 pm, (EA) 22-25 pm. Isopolar. Exine 1 pm thick, sexine tectate, echinate, spines 2 pm (Ybert et al., 2016).
Ecological procedence: perennial creeper well represented in hygrophilous forest vegetation of Iberá Wetlands (Arbo & Tressens, 2002), as well as in forests, savannas, riparian banks of rivers and streams of South America (Anton & Zuloaga, 2021).
Senecio bonariensis Hook. & Arn., J. Bot. (Hooker) 3: 340. 1841. Fig. 3C.
Morphology: Tricolporate pollen grain, radial symmetry. Circular in (PA) 28-40 pm, prolate in (EA) 16-35 pm. Isopolar. Exine 2-5 pm thick. Sexine tectate, echinate, spines 2-3 pm (Markgraf & D'Antoni, 1978).
Ecological procedence: Senecio L. is well represented in the natural vegetation of the Iberá Wetlands in marsh and grassland, corresponding to local taxa (Arbo & Tressens, 2002) and present in Chacoan, Paranaense and Pampean floristic provinces (Anton & Zuloaga, 2021).
Cyperaceae (Liliopsida, Poales)
Cyperus rotundus L., Sp. Pl. 1: 45. 1753. Fig. 3D.
Morphology: Monoporate pollen grain and radial symmetry. Heteropolar, prolate, (PA) 27-37 pm, (EA) 16-24 pm. Exine 1.5 pm thick, ornamentation granular (Fernandez, 1987).
Ecological procedence: perennial marsh grass well represented in the natural vegetation of the Iberá Wetlands (Arbo & Tressens, 2002).
Ericaceae (Magnoliopsida, Ericales)
Empetrum aff. rubrum Vahl ex Willd., Sp. Pl. 4: 713. 1806. Fig. 3E, F.
Morphology: Tetrad with tricolporate pollen grain, radial symmetry. Small, circular-subcircular, (EA) 19-22 pm. Isopolar. Exine 2 pm thick (Markgraf & D'Antoni, 1978).
Ecological procedence: subshrub represented in marshes of the natural vegetation of the Iberá Wetlands (Camevalli, 1994; Arbo & Tressens, 2002).
Haloragaceae (Magnoliopsida, Saxifragales)
Myriophyllum aquaticum (Vell.) Verdc., Kew Bull. 28: 36. 1973. Fig. 3G.
Morphology: Tetrazonoporate pollen grain with radial symmetry. Subcircular-angular amb, oblate to spheroidal (PA) 29-38 pm, (EA) 34-41 pm. Pores 4-5 pm diam., protrudent, with thickening of the endexine. Exine 1.5 pm thick. Sexine tectate, scabrate (Diez Dapena, 1988).
Ecological procedence: perennial aquatic herb well represented in the natural vegetation of the
Iberá Wetlands (Arbo & Tressens, 2002) also present in central-northern region of Argentina (Anton & Zuloaga, 2021).
Iridaceae (Liliopsida, Asparagales)
Cypella herbertii Herb., Bot. Mag. 53: sub t. 2637. 1826. Fig. 3H.
Morphology: Monosulcate pollen grain and bilateral symmetry. Heteropolar, suboblate, (PA) 40-54 pm, (EA) 45-61 pm. Sulcus 35-54 pm long. Exine 1-2.2 pm thick, sexine semitectate and microreticulate (Salgado, 2006).
Ecologic procedence: bulbous herbaceous local species in marsh areas (Boelcke, 1992; Arbo & Tressens, 2002). Endemic of Pampean and Paranaense floral provinces (Anton & Zuloaga, 2021).
Leguminosae, Mimosoideae (Magnoliopsida, Fabales)
Calliandra parvifolia (Hook. & Arn.) Speg., Revista Argent. Bot. 1: 193. 1926. Fig. 3I.
Morphology: Polyads 185 x 112 pm diam. usually of 8 pollen grains. Exine 2 pm thick, rugulate (Markgraf & D'Antoni, 1978).
Ecological procedence: extra-local shrub represented in the natural vegetation of Corrientes province in forests of S. balansae and M. balansae (Fontana, 2018).
Anadenanthera colubrina (Vell.) Brenan, Kew Bull. 10: 182. 1955. Fig. 3J.
Morphology: Polyads spheroidal to ellipsoidal of 12 pollen grains irregularly arranged, or of 16 pollen grains regularly arranged. Exine 1.3 pm thick. Verrucate, 1 pm diam. warts (Caccavari & Dome, 2006).
Ecological procedence: extra-local tree represented in forests of S. balansae and M. balansae of Corrientes province (Fontana, 2018).
Prosopis alba Griseb., Abh. Konigl. Ges. Wiss. Gottingen 19: 131. 1874. Fig. 3K.
Morphology: Tricolporate pollen grain of radial symmetry. Small to medium size, prolate, (PA) 22-36 pm, (EA) 18.5-33 pm. Isopolar. Long colpi 17-30 pm and pores 4-6 pm diam. Exine 1-2 pm thick, sexine tectate, scabrate (Fernandez Pacella et al., 2014b).
Ecological procedence: extra-local tree represented in forests of S. balansae and M. balansae of Corrientes (Boelcke, 1992; Fontana, 2018).

Fig. 3. Pollen of Gutierrezia, Mikania, Senecio, Cyperus, Empetrum, Myríophyllum, Cypella, Calliandra, Anadenanthera, Prosopis, Senegalia, Trichilia, Myrcianthes, and Plinia. A: Gutierrezia resinosa, equatorial view in optical section (100x). B: Mikania cordifolia, polar view in optical section (100x). C: Senecio bonariensis, polar view in optical section (100x). D: Cyperus rotundus, equatorial view in optical section (100x). E: Empetrum aff. Rubrum, poliade in optical section (100x). F: Poliade in superior focus (100x). G: Myriophyllum aquaticum, polar view in superior focus (100x). H: Cypella herbertii, polar view in optical section (100x). I: Calliandra parvifolia, poliade (100x). J: Anadenanthera colubrina, poliade (100x). K: Prosopis alba, polar view in superior focus (100x). L: Senegalia bonariensis, poliade (100x). M: Trichilia elegans, equatorial view in optical section (100x). N: Equatorial view in superior focus (100x). O: Myrcianthes pungens, polar view in superior focus (100x). P: Plinia rivularis, polar view in superior focus (100x). Scales= 10 pm.
Senegalia bonariensis (Gillies ex Hook. & Arn.) Seigler & Ebinger, Phytologia 88: 50. 2006. Fig. 3L.
Morphology: Polyads of 16 pollen grains with regular arrangement, major axis (34-42 pm), minor axis (22-30 pm). Exine 1.55-2.55 pm thick. Sexine tectate. Infratectum with columellae 0.5-0.9 pm long. Ornamentation slightly granulate (Caccavari & Dome, 2001).
Ecological procedence: tree or shrub present in hygrophilous forests of the Iberá Wetlands (Boelcke, 1992; Fontana, 2018).
Meliaceae (Magnoliopsida, Sapindales)
Triehilia elegans A. Juss., Fl. Bras. Merid. (quarto ed.) 2: 79, t. 98. 1829. Fig. 3M, N.
Morphology: Tetracolporate pollen grain, radial symmetry. Small size, quadrangular amb (PA) 17-25 pm, subprolate (EA) 14-21 pm. Isopolar. Colpi 15-22 pm long, lalongate endoapertures with ring thickening 1.4-3 pm thick. Exine 1.4 pm thick and microreticulate ornamentation (Oliveira & Santos, 2014).
Ecological procedence: extra-local tree represented in forests of S. balansae and M. balansae of the natural vegetation of Corrientes (Fontana, 2018) and noreastern Argentina (Anton & Zuloaga, 2021).
Myrtaceae (Magnoliopsida, Myrtales)
Myrcianthes pungens (O. Berg) D. Legrand, Bol. Fac. Agron. Univ. Montevideo 101: 52. 1968. Fig. 3O.
Morphology: Parasintricolporate pollen grain, radial symmetry. Small size, triangular, oblate, (PA) 9-17 pm, (EA) 12-21 pm. Isopolar. Narrow or wide colpi an apocolpial field or island, of triangular form. Apocolpial fields with edges and are of different sizes in both poles. Endoapertures 1.4-2 pm. Exine 0.7-3 pm thick, sexine semitectate, microreticulate (Acevedo & Anzótegui, 1998).
Ecological procedence: the natural vegetation of Corrientes province in forests of S. balansae and M. balansae (Boelcke, 1992; Fontana, 2018). Native of North-central region of Argentina (Anton & Zuloaga, 2021).
Plinia rivularis (Cambess.) Rotman, Bol. Soc. Argent. Bot. 24: 195. 1985. Fig. 3P.
Morphology: Parasyntricolporate pollen grain, radial symmetry. Triangular amb, oblate, (PA) 13-17 pm, (EA) 19-28 pm. Isopolar. Linear colpi, less than 1 pm wide, lalongate endoapertures 1 x 2.3 pm. Exine 0.7-2 pm thick. Sexine tectate. Psilate (Acevedo & Anzótegui, 1998).
Ecological procedence: tree well represented in hygrophilous forests of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002).
Exclusive of Entre Ríos, Corrientes and Misiones belonging to the Paranaense floristic province (Anton & Zuloaga, 2021).
Nyctaginaceae (Magnoliopsida, Caryophyllales)
Pisonia aculeata L., Sp. Pl. 2: 1026. 1753. Fig. 4A, B.
Morphology: Tricolporate pollen grain of radial symmetry. Medium size, prolate, (PA) 3437 pm, (EA) 24-27 pm. Isopolar. Colpi 32-35 pm long. Pores 2-3 pm diam. Exine 1-1.5 pm thick, sexine semitectate, microreticulate (Oliveira & Santos, 2014).
Ecological procedence: shrub or creeper species present in natural forests of Schinopsis balansae Engl. and Myracrodruon balansae (Engl.) Santin in Corrientes province (extra-local taxon) (Carnevalli, 1994; Fontana, 2018), and northern region of Argentina (Anton & Zuloaga, 2021).
Onagraceae (Magnoliopsida, Myrtales)
Ludwigia peploides (Kunth) P.H. Raven, Reinwardtia 6: 393 1963[1964]. Fig. 4C.
Morphology: Tricolporate pollen grain, radial symmetry. Large size, subtriangular amb, subprolate, (PA) 94-108 pm, (EA) 74-86 pm. Isopolar. Colpi 18 x 4 pm, endoapertures 20 x 16 pm protrudent-vestibuled. Exine 3-4 pm thick. The surface of the ridges is rugulate-striated. The area between the ridges has an uneven coarse rugulate appearance, due to the presence of irregular striae (Cecotti Álvarez et al., 2017).
Ecological procedence: aquatic herbaceous taxon well represented in fresh water bodies of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002). Native of central-northern region of Argentina (Anton & Zuloaga, 2021).
Poaceae (Liliopsida, Poales)
Eehinoehloa crus-galli (L.) P. Beauv., Ess. Agrostogr. 1: 53, 161, 169, pl. 11, f. 2. 1812. Fig. 4D.
Morphology: Monoporate pollen grain and radial symmetry. Spheroidal amb, (PA) 46-49 pm, (EA) 46-49 pm. Heteropolar. Pore 2 pm in diameter, with annulus 1 pm thick. Exine 2 pm thick. Sexine tectate, psilate to scabrate (Fernandez Pacella & Canteros, 2014).
Ecological procedence: herbaceous species in marsh and grasslands of natural vegetation of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002) and widely distributed in Argentina (Anton & Zuloaga, 2021).
Echinoehloa polystaehya (Kunth) Hitchc., Contr. U.S. Natl. Herb. 22: 135. 1920. Fig. 4E.
Morphology: Monoporate pollen grain and radial symmetry. Spheroidal amb, (PA) 53-57 pm, (EA) 60-62 pm. Heteropolar. Pore with annulus 2 pm in diameter. Exine 2 pm thick, sexine tectate, psilate to scabrate (Fernandez Pacella & Canteros, 2014).
Ecological procedence: herbaceous taxon, local of marsh and grasslands in the natural vegetation of the Iberá Wetlands (Arbo & Tressens, 2002). Extra-local species present in Paranense, Chacoan and Pampean floral provinces of Argentina (Anton & Zuloaga, 2021).
Hymenachne pernambucensis (Spreng.) Zuloaga, Amer. J. Bot. 90: 817. 2003. Fig. 4F.
Morphology: Monoporate pollen grain, radial symmetry. Prolate-spheroidal, (PA) 42-44 pm, (EA) 36-39 pm. Heteropolar. Pore 3 pm in diameter. Exine 2 pm thick. Sexine tectate, psilate to scabrate (Fernandez Pacella & Canteros, 2014).
Ecological procedence: H. pernambucensis local genus is well represented in marsh and grasslands (Arbo & Tressens, 2002). Present in Paranense and Chacoan floral provinces of Argentina (Anton & Zuloaga, 2021).
Imperata brasiliensis Trin., Mém. Acad. Imp. Sci. St.-Pétersbourg, Sér. 6, Sci. Math. 2: 331. 1832. Fig. 4G.
Morphology: Monoporate pollen grain, radial symmetry. Spheroidal amb, (PA) 21-37 pm, (EA) 18-33 pm. Heteropolar. Pore 1-4 pm diameter with a ring 1 to 6 pm thick. Exine 1-3 pm thick, tectate, psilate to scabrate (Fernandez Pacella & Canteros, 2014).
Ecological procedence: herbaceous species well represented in marsh and grassland areas of the natural vegetation of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002).
Polygalaceae (Magnoliopsida, Fabales)
Polygala leptocaulis Torr. & A. Gray, Fl. N. Amer. 1: 130. 1838. Fig. 4H.
Morphology: Pantozonocolporate pollen grain with radial symmetry. Subcircular amb (PA) 20-30 pm, subprolate amb (EA) 18-21 pm. Isopolar. Colpi 8-15, 17-25 pm long. Lalongate endoaperture 2 x 4 pm. Exine 0.7-2.5 pm thick. Sexine tectate. Psilate (Cuadrado, 1998c).
Ecological procedence: herbaceous species well represented in marshes and grasslands of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002).
Polygonaceae (Magnoliopsida, Caryophyllales)
Polygonum acuminatum Kunth, Nov. Gen. Sp. (quarto ed.) 2: 178. 1818. Fig. 4I, J.
Morphology: Triporate pollen grain with radial symmetry. Circular amb, (EA) 59-62 pm. Exine 6 pm thick. Sexine 1.5-2 pm thick, reticulate, walls 1.5 pm thick, simplicolumellate, lumen 10 pm wide (Ybert et al., 2018).
Ecological procedence: herbaceous species well represented in hygrophilous forests of the Iberá Wetlands (Fontana, 2018).
Polygonum convolvulus L., Sp. Pl. 1: 364. 1753. Fig. 4K.
Morphology: Triporate pollen grain with radial symmetry. Circular-subcircular amb, (EA) 39-42 pm. Isopolar. Exine 3 pm thick, reticulate, walls 1 pm thick, simplicolumellate, irregular lumen 2 x 2 pm wide (Ybert et al., 2018).
Ecological procedence: herbaceous species represented in hygrophilous forests of the Iberá Wetlands (Fontana, 2018).
Sapindaceae (Magnoliopsida, Sapindales)
Serjania perulacea Radlk., Consp. Sect. Sp. Serjan.: 11. 1874. Fig. 4L, M.
Morphology: Hemisyntricolporate pollen grain, radial symmetry. Subtriangular amb, oblate, (PA) 8-40 pm, (EA) 6-58 pm. Heteropolar. Colpi 1-2 pm wide. Exine 0.7-2.5 pm thick, sexine semitectate, microreticulate (Anzótegui & Ferrucci, 1998).
Ecological procedence: extra-local perennial creeper represented in the forests of Schinopsis balansae and Myracrodruon balansae, natural vegetation of Corrientes (Fontana, 2018) and other provinces of northern Argentina: Chaco, Formosa, Jujuy, Salta (Anton & Zuloaga, 2021).

Fig. 4. Pollen of Pisonia, Ludwigia, Echinochloa, Hymenachne, Imperata, Polygala, Polygonum, Serjania, Thinouia, Chrysophyllum, and Typha. A: Pisonia aculeate, equatorial view in optical section (100x). B: Equatorial view in superior focus (100x). C: Ludwigia peploides, polar view in optical section (100x). D: Echinochloa crus-galli, equatorial view in superior focus (100x). E: Echinochloa polystachya, equatorial view in superior focus (100x). F: Hymenachne pernambucensis, equatorial view in optical section (100x). G: Imperata brasiliensis, equatorial view in superior focus (100x). H: Polygala leptocaulis, equatorial view in superior focus (100x). I: Polygonum acuminatum, polar view in superior focus (100x). J: Polar view in optical section (100x). K: Polygonum convolvulus, polar view in superior focus (100x). L: Serjania perulacea, polar view in inferior focus (100x). M: Polar view in superior focus (100x). N: Thinouia mucronata, polar view in superior focus (100x). O: Chrysophyllum marginatum, equatorial view in optical section (100x). P: Typha domingensis, equatorial view in superior focus (100x). Scales= 10 pm.
Thinouia mucronata Radlk., Sitzungsber. Math.-Phys. Cl. Konigl. Bayer. Akad. Wiss. München 8: 282. 1878. Fig. 4N.
Morphology: Tricolporate pollen grain, radial symmetry. Subtriangular amb, oblate, (PA) 1127 pm, (EA) 23-27 pm. Sides slightly convex
to substraight. Isopolar. Colpi (1.4-4 pm wide). Lalongate endoapertures 1.4 x 4.3 pm. Exine 1.42.1 thick. Psilate to slightly scabrate (Anzótegui & Ferrucci, 1998).
Ecological procedence: local perennial creeper well represented in hygrophilous forest vegetation of the Iberá Wetlands (Arbo & Tressens, 2002; Boelcke, 1992) and in northern Argentina (Anton & Zuloaga, 2021).
Sapotaceae (Magnoliopsida, Ericales)
Chrysophyllum marginatum (Hook. & Arn.) Radlk., Act. Occ. Exp. Univ. Anvers Coin. Exp. Int. Hort.: 170. 1887. Fig. 4O.
Morphology: Tetracolporate pollen grain with radial symmetry. Subtriangular amb, prolate, (PA) 20-24 pm, (EA) 14-16 pm. Isopolar. Narrow colpi ±14 pm long. Endoapertures 2 x 3 pm with an annular thickening of 2 pm given by protruding endexine. Exine 3 pm thick, columellate infratectal layer observed. At the poles, the exine is 2 pm, the columellae being longer than the thick of the tectum and the nexine 1 pm; at the equator the sexine thins, until approximately 1 pm and the nexine increases its thickness to 2 pm. Surface scabrate (Cuadrado, 1998b).
Ecological procedence: shrub or small tree well represented in forests of the Iberá Wetlands and Norht-central region of Argentina (Arbo & Tressens, 2002; Anton & Zuluoga, 2021).
Typhaceae (Liliopsida, Poales)
Typha domingensis Pers., Syn. Pl. 2: 532. 1807. Fig. 4P.
Morphology: Monoporate pollen grain, radial symmetry. Spheroidal amb, (PA) 17-20 pm, (EA) 17-20 pm. Heteropolar. Exine 2.5 pm thick. Sexine columellate, wall 0.25 pm thick, reticulate (Alonso, 2014).
Ecological precedence: robust emergent aquatic herbaceous local taxon well represented in marshes of natural vegetation of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002).
Ulmaceae (Magnoliopsida, Rosales)
Phyllostylon rhamnoides (J. Poiss.) Taub., Oesterr. Bot. Z. 11: 409. 1890. Fig. 5A, B.
Morphology: Tetra-pentazonoporate pollen grain, radial symmetry. Spheroidal, (PA) 36-39 pm, (EA) 36-39 pm. Isopolar. Circular pores 1-3 pm in diam., some of them arranged in a subequatorial plane. Exine 1 pm thick. Psilate to verrucate (Anzótegui & Mautino, 2001b).
Ecological procedence: extra-local tree well represented in forests of S. balansae and M. balansae of Corrientes province (Boelcke, 1992;
Fontana, 2018). Native of Chacoan and Yungas floristic provinces of Argentina (Anton & Zuloaga, 2021).
Umbeliferae (Magnoliopsida, Apiales)
Eryngium elegans Cham. & Schltdl., Linnaea 1: 348. 1826. Fig. 5C.
Morphology: Tricolporate pollen grain, radial symmetry. Prolate, (PA) 28-36 pm, (EA) 18-23 pm. Isopolar. Narrow and long colpi, relatively large pore. Exine 1 pm thick. Sexine semitectate, perforate, microreticulate (Alonso, 2014).
Ecological procedence: local herb in marshes of the Iberá Wetlands (Boelcke, 1992; Arbo & Tressens, 2002), and elsewhere in Corrientes (Fontana, 2018) and North-central Argentina (Anton & Zuloaga, 2021).
Vitaceae (Magnoliopsida, Vitales)
Cissus verticillata (L.) Nicolson & C.E. Jarvis, Taxon 33: 727. 1984. Fig. 5D-F.
Morphology: Tricolporate pollen grain, radial symmetry. Subtriangular amb, subprolate, (PA) 75-77 pm, (EA) 58-60 pm. Isopolar. Colpi long, maximum width of 4.5 pm and ribs 2.8-4.5 pm thick at the equator. Lalongate endoapertures 5-8 x 3-4 pm to circular 3 pm diam. Exine 2-4 pm thick, reticulate (Anzótegui & Caccavari, 2001).
Ecological procedence: perennial creeper represented in hygrophilous forest of the natural vegetation of the Iberá Wetlands (Fontana, 2018) and in central-northern of Argentina (Anton & Zuloaga, 2021).
The nine recorded species with spores are listed alphabetically by family:
Anthocerotaceae (Anthocerotopsida, Anthocerotales)
Anthoceros aff. lamellatus Steph., Sp. Hepat. (Stephani) 5: 1000. 1916. Fig. 5G, H.
Morphology: Trilete spore. Polar view subtriangular. Laesura with straight rays reaching the equator. Exospore 3.5 pm thick, smooth inner proximal surface, bacullate distal surface and equatorial amb. Size: 48-76 pm (Gradstein, 2018).
Ecological procedence: cosmopolitan genus is represented on compact rock surfaces exposed to light (Peñaloza-Bojacá et al., 2020).
Cyatheaceae (Polypodiopsida, Cyatheales)
Cyathea aff. atrovirens (Langsd. & Fisch.) Domin,
Rozpr. Kral. Ceske Spolecn. Nauk, Tr. Mat.-Prir. 2: 262. 1929. Fig. 5I.
Morphology: Trilete spore. Polar view triangular. Laesura 35-40 pm long. Exospore 1.4 pm thick. Psilate or scabrate. Size: 43-53 pm (Contreras-Duarte et al., 2006).
Ecological procedence: tree to shrub and herb on soils and rock substrate (rupicolous species) on roadsides, ravines, in abandoned fields, swamps and secondary forests of forests of the Iberá Wetlands (Arbo & Tressens, 2002) and in Corrientes and Misiones provinces (Anton & Zuloaga, 2021).
Lycopodiaceae (Lycopodiopsida, Lycopodiales)
Phlegmariurus aff. mandiocanus (Raddi) B. 0llg., Rodriguésia 63: 480. 2012. Fig. 5J.
Morphology: Trilete spore. Polar view subtriangular, with rounded angles. Lesura with straight rays reaching to the equator. Exospore 1.6 pm thick. Psilate on proximal face. Size: 28-38 pm. Illustrations of this taxon by Sersic (1983) and comparison with other species of the genus consulted in Rincón et al. (2014).
Ecological procedence: epiphyte present in hygrophilous forests of Yungas and Paranaense floral provinces in Argentina (Anton & Zuloaga, 2021; Windisch et al, 2015).
Notothyladaceae (Anthocerotopsida, Notothyladales)
Phaeoceros bulbiculosus (Brot.) Prosk., Rapp. Comm., VIII Congr. Int. Bot.: 69. 1954. Fig. 5K.
Morphology: Trilete spore. Polar view subtriangular. Laesura with straight rays reaching the equator. Exospore 2.3 pm thick. Granular/ gemmate sculpture. Size: 32-38 pm. Illustrated from Argentina (Morbelli et al., 2010).
Ecological procedence: cosmopolitan genus is represented on compact, moist soil, and on rock in moist environments, exposed or partially shaded microhabitats (Peñaloza-Bojacá et al., 2020).
Phaeoceros tenuis (Spruce) Hassel, Veroff. Geobot. Inst. ETH Stiftung Rübel Zürich 91: 303. 1986. Fig. 5L.
Morphology: Trilete spore. Polar view subtriangular. Laesura with straight rays reaching the equator. Exospore 3.5 pm thick. Granular. Size: 48-76 pm (Prieto & Quattrocchio, 1993).
Ecological procedence: cosmopolitan genus is represented on compact, moist soil, and on rock in moist environments, exposed or partially shaded microhabitats (Peñaloza-Bojacá et al., 2020).
Pteridaceae (Polypodiopsida, Polypodiales)
Jamesonia flexuosa (Kunth) Christenh., Phytotaxa 19: 21. 2011. Fig. 5M.
Morphology: Trilete spore. Polar view subtriangular, with rounded angles. Sinuous laesura, with small coalescent ridges, reaching to the inner margin of the cingulum. Exospore including the cingulum is 5.5 pm thick. Size: 56-71 pm (Contreras-Duarte et al., 2006; Della & Prado, 2020).
Ecological procedence: this taxon is part of the natural vegetation of the Iberá Wetlans (Arbo & Tressens, 2002) and occurs in humid places inside of cloud forest from southern Mexico to Bolivia, and north and southeast of Brazil to Uruguay (Della & Prado, 2020; Della et al, 2020).
Pellaea sp. Fig. 5N
Morphology: Trilete spore. Polar view triangular. Rays of the laesurae straight, 2/3 of radius to almost the internal margin of cingulum. Exospore cingulum surrounds the spore, 5.4 pm thick. Central part of distal face verrucate/rugulate, low warts occasionally joined forming loins. Size: 37-48 pm.
Comparisons: few specimens recorded herein are similar to Pellaea cordifolia (Sessé & Moc.) A.R. Sm. (Arreguín-Sánchez et al., 1996; Pérez-Jiménez et al., 2020) and Pellaea ovata (Desv.) Weath. (Gómez et al, 2013) differing in having smooth proximal and less ornamented distal face.
Ecological procedence: this genus is including terrestrial or rupicolous species, some of humid shaded areas of hygrophilous forests and others tolerate slight exposition to light in open areas. Particularly, Pellaea cordifolia and P ovata (Hirai & Prado, 2021) are known in South America (Anton & Zuloaga, 2021; Arbo & Tressens, 2002).
Selaginellaceae (Lycopodiopsida, Selaginellales)
Selaginella aff. marginata (Humb. & Bonpl. ex Willd.) Spring, Flora 21: 194. 1838. Fig. 5O. Morphology: Trilete microspore. Polar view subtriangular. Laesura with straight rays reaching to the equator. Exospore thin 1-2 pm thick. Baculate with less frequent spinose and verrucate ornamentation. Size: 17-37 pm. Micro-megaspores belonging to this taxon were described from

Fig. 5. Palynomorphs of Phyllostylon, Eryngium, Cissus, Anthoceros, Cyathea, Phlegmariurus, Phaeoceros, Jamesonia, Pellaea, and Sellaginella. A: Phyllostylon rhamnoides, general view in superior focus (100x). B: General view in optical section (100x). C: Eryngium elegans, equatorial view in optical section (100x). D: Cissus verticillata, equatorial view in optical section (100x). E: Equatorial view in superior focus (100x). F: Equatorial view where apertures are appreciated (100x). G: Anthoceros aff. Lamellatus, polar view in optical section (100x). H: Polar view where the trilete is appreciated (100x). I: Cyathea aff. Multiflora, polar view in superior focus (100x). J: Phlegmariurus aff. Mandiocanus, polar view in superior focus (100x). K: Phaeoceros bulbiculosus, polar view in optical section (100x). L: Phaeoceros tenuis, polar view in superior focus (100x). M: Jamesonia flexuosa, polar view in superior focus (100x). N: Pellaea sp., polar view in optical section (100x). O: Selaginella aff. Marginata, polar view where the trilete is appreciated (100x). P: Sphagnum sp., polar view in optical section (100x). Scales= 10 pm.
Argentina (Morbelli, 1977; Morbelli et al., 2001) and from Brazil (Lorscheitter et al, 1998).
Ecological procedence: perennial herbaceous represented in Selaginella sellowii Hieron. grass of the natural vegetation of Corrientes (Fontana, 2018).
Sphagnaceae (Sphagnopsida, Sphagnales)
Sphagnum sp. Fig. 5P.
Morphology: trilete spore. Polar view subtriangular, with rounded angles. Rays of the laesurae reach the equator. Exospore 2-4.5 pm thick. Finely rugged. Size: 20-26 pm (Fuertes & Rodríguez, 2009).
Ecological procedence: moss well represented in marshes of the natural vegetation of the Iberá System (Arbo & Tressens, 2002).
Discusión
Local and extra- local taxa and plant associations during the Holocene
The first stage of the Mid Holocene, between 6990 and 5800 cal year BP is characterized mostly by local taxa such as Imperata brasiliensis, Echinochloa crus-galli, Echinochloa polystachya, Hymenachne pernambucensis, Cyperus rotundus, Crinum americanum, Cypella herbertii, Empetrum aff. rubrum, Polygala leptocaulis, Myriophyllum aquaticum and Ludwigia peploides, which integrate marsh grasslands and hygrophilous communities suggesting locally humid conditions in the studied area (Fernandez Pacella & Lara, 2019) (Fig. 6).
A later stage of the Mid Holocene, between 5800 and 5141 cal year BP is characterized by local taxa of Poaceae, Cyperaceae, Typha dominguensis, Sphagnum sp., representing a characteristic wetland association. The presence of Typha suggests waterlogged or flooded soils with slow-moving water and poor drainage conditions (Cabrera, 1976; Carnevalli, 1994). These plant communities, characteristic of lakes, swampy depressions and low floodplains, would indicate for this later stage of the Mid Holocene, sub-humid to humid conditions (Fernandez Pacella & Lara, 2019) (Fig. 6).
Between 5141 and 3506 cal year BP was characterized by a prevalence of local herbaceous vegetation composed of Anthemis cotula, Conyza pampeana, Baccharis trimera, Gutierrezia resinosa, Bidens subalternans, Gaillardia megapotamica, Senecio bonariensis, Amaranthus muricatus, Eryngium elegans and Poaceae, characteristic of psammophilous herbaceous steppe (Fig. 6). The presence of Chenopodiaceae suggests periodic desiccation of the water bodies (Tonello & Prieto, 2008). These results suggest dry environmental conditions (Fernandez Pacella et al, 2011; Fernandez Pacella & Lara, 2019). During this stage, different extra-local species are documented, which indicate the beginning of the development of various types of forests known today as Forests of Schinopsis balansae and Myracrodruon balansae ("quebrachal") of the humid Chaco phytogeographic region, better represented in Chaco, Formosa and northern Santa Fe provinces, with impoverished remains in the NW of Corrientes (Fontana, 2018). The main species documented herein are Anadenanthera colubrina, Myracrodruon balansae, Calliandra parvifolia, Myrcianthes pungens, Phyllostylon rhamnoides, Pisonia aculeata, Prosopis alba, Schinopsis balansae, Schinus longifolia, Trichilia elegans, Justicia brasiliana and Serjania perulacea. This type of forest develops in highest topographic areas free of floods characterized by soils with lower moisture gradients (well-drained) and a higher proportion of fine particles (Maldonado & Hohne, 2006; Morello & Rodríguez, 2009). Among these taxa, Anadenanthera was recorded from the Miocene to Pliocene in Patagonia and its last record is extended into the Holocene of the Iberá Wetland (Fernandez Pacella, 2015).

Fig. 6. Floristic representation of local and extra-local taxa and plant associations during the Holocene. LTmg-hc: local taxa-marsh grasslands and hygrophilous communities. LTwa: local taxa-wetland association. LTphs: local taxa-psammophilous herbaceous steppe. LThf: local taxa-hygrophilous forest. ExT: extra-local taxa.
From 3484 cal year BP until the present (Late Holocene) the palynologic record shows a prevalence of herbaceous local vegetation composed of marshy taxa and wetland association (Fig. 6). Local taxa from the "Hygrophilous Forest", represented by Senegalia bonariensis, Celtis iguanaea, Polygonum acuminatum, Polygonum convolvulus, Cissus verticillata, Plinia rivularis, Tecoma stans, Thinouia mucronata, Chrysophyllum marginatum, Mikania cordifolia, Cyathea atrovirens, Jamesonia flexuosa, Phlegmariurus mandiocanus, Pellaea sp., Phaeoceros tenuis and Phaeoceros bulbiculosus also documented in variable frequency (Fernandez Pacella et al, 2011; Fernandez Pacella & Lara, 2019). This Hygrophilous Forest developed in this interval, forms a strip that extends along small floodplain valleys close to "madrejones" or ponds with reservoirs, sometimes superimposed on the grasslands (Eskuche, 2004; Garcia et al, 2013). It is characterized by low to medium trees in less dense forests that allows enough light input for the establishment of herbaceous communities as part of the understory vegetation. The interpretation of "humid forests community" is clearly supported by the abundance of epiphytes and creeping taxa giving an idea of a jungle type environment (Carnevalli, 1994; Fontana, 2018).
Comparison with modern vegetation
All the species described herein are still part of the flora of Corrientes. For example, Selaginella aff. marginata present in the Mburucuyá National Park (Meza Torres et al., 2013) constitutes the herbaceous community of "Selaginella sellowii grass" that grows on eroded soils in the open forests of Schinopsis balansae and Myracrodruon balansae of north-western Corrientes (Bauni & Homberg, 2015; Fontana, 2018). These forests characterized by tree and shrubby forms herein documented as well (Anadenanthera colubrina, Myracrodruon balansae, Calliandra parvifolia, Myrcianthes pungens, Phyllostylon rhamnoides, Pisonia aculeata, Prosopis alba, Schinopsis balansae, Trichilia elegans, Justicia brasiliana, Serjania perulacea).
Syagrus romanzoffiana and Schinus longifolia represent the extensive palm groves particularly on the western coast of the Paraná River in the NW of Corrientes. They develop in floodplain valleys of some streams and depressed areas with soils bearing a high content of fine materials (silt and clays), mixed with sand, brought by the river, generally subjected to frequent, non-permanent floods (Fontana, 2018).
The local herbaceous taxa Imperata brasiliensis, Echinochloa crus-galli, Echinochloa polystachya, Hymenachne pernambucense, Cyperus rotundus, Eryngium elegans, Cypella herbertii, Myriophyllum aquaticum, Empetrum aff. rubrum, Polygala leptocaulis, Ludwigia peploides, Typha dominguensis, Celtis iguanaea, Anthemis cotula, Conyza pampeana, Baccharis trimera, Mikania cordifolia, Gutierrezia resinosa, Bidens subalternans, Gaillardia megapotamica, Senecio bonariensis, Amaranthus muricatus and Sphagnum sp., are currently part of the hygrophilous communities of marsh- grasslands, wetlands and psammophilous herbaceous steppe of the Iberá Wetland (Arbo & Tressens, 2002).
Senegalia bonariensis, Polygonum acuminatum, Polygonum convolvulus, Cissus verticillata, Plinia rivularis, Tecoma stans, Thinouia mucronata, Chrysophyllum marginatum, Cyathea atrovirens, Jamesonia flexuosa, Phlegmariurus saururus,
Pellaea sp., Phaeoceros tenuis and Phaeoceros bulbiculosus, represent varied habits from tree to epiphyte of the Hygrophilous Forests in the W, N and center of Corrientes (Camevalli, 1994; Fontana, 2018).
Conclusión
The 55 pollen and spore types described and illustrated in this contribution reaffirm diversified floras existed during the Mid-Late Holocene in Corrientes. Of them, 46 pollen grains correspond to 27 families of angiosperms and 9 trilete spore-types of ferns, lycophytes and bryophytes, obtained from six lakes of this wetland. Forty-two (42) taxa of these lived in the Iberá wetland (local taxa), whereas 13 extra-local ones would have arrived chiefly through air currents. This ecologic/procedence differentiation of species and their relative frequency per sample in each core (pollen diagrams) presented by Fernandez Pacella et al. (2011), Fernandez Pacella (2013), Fernandez Pacella & Lara (2019) was applied to interpret environmental characteristics and the evolution of the Iberá vegetation. Therefore, the identification of pollen grains up to species level enhances paleoenvironmental reconstructions based on more accurate ecological information and geographic distribution. Some local species as, considered in this studied region, were temperate-environmentally restricted and, for example, during the first stage of the Late Holocene, characteristic species of psammophilous herbaceous steppe were documented. On the other hand, in a later stage of the Late Holocene, several species appeared indicating the beginning of the development of "Hygrophilous Forest". Instead, other species such as grasses are dominant throughout the Holocene in Corrientes. This work broadens the knowledge of the palynological flora of northeastern Argentine and, will help to differentiate the local vegetation from the extra-local in future interpretations.
Authors contribution
Both authors have jointly and equally carried out the data collection, interpretation and writing of the manuscript.
Acknowledgements
The authors wish to express their gratitude to Prof. Oscar Canteros performing the chemical processing of samples, to Teodoro Roberto (technical) accompanying us in the different journeys.
Funding: This work was supported by Secretaría General de Ciencia y Técnica, Universidad Nacional del Nordeste (PI 18Q006).
Bibliography
ACEVEDO, T. L. & L. M. ANZÓTEGUI. 1998. Myrtaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. I: 67-80. EUDENE, Corrientes.
ALONSO, U. C. 2014. Palinoteca de Plantas Vasculares Acuáticas para el Análisis Polínico en Paleolimnología. Graduate Thesis. Universidade da Coruña, España.
ANTON, A. M. & F. O. ZULOAGA (dir). Flora Argentina [online]. Available in: http://www. floraargentina.edu.ar [Access: 25 October 2021]. ANZÓTEGUI, L. M. 2001. Anacardiaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. II: 19-26. EUDENE, Corrientes.
ANZÓTEGUI, L. M. & S. S. GARRALLA. 1985. Estudio palinológico de la Formación Paraná (Mioceno superior), (Pozo Josefina), Provincia de Santa Fe, Argentina. I parte: Descripciones sistemáticas. Facena 6: 101-177.
ANZÓTEGUI, L. M. & M. S. FERUCCI. 1998. Sapindaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. I: 95-110. EUDENE, Corrientes.
ANZÓTEGUI, L. M. & M. A. CACCAVARI. 2001. Vitaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol: 141-147. EUDENE, Corrientes.
ANZÓTEGUI, L. M. & L. R. MAUTINO. 2001a. Celtidaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. II: 61-64. EUDENE, Corrientes
ANZÓTEGUI, L. M. & L. R. MAUTINO. 2001b. Ulmaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. II: 137-139. EUDENE, Corrientes.
APG IV. 2017. Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Checklist dataset [online]. Available in: https://doi.org/10.15468/fzuaam accessed via GBIF. org
ARBO, M. & S. TRESSENS. 2002. Flora del Iberá: Corrientes, Argentina. EUDENE, Corrientes.
ARREGUÍN-SÁNCHEZ, M., R. FERNÁNDEZ NAVA, R. PALACIOS CHÁVEZ & D. L. QUIROZ GARCÍA. 1996. Morfología de las esporas de Pteridófitas Isospóreas del Estado de Querétaro, México (parte a). Polibotánica 2: 10-60.
BAUERMANN, S. G., A. CARDOSO PACHECO EVALDT, J. R. ZANCHIN & S. A. DE LORETO BORDIGNON. 2010. Diferenciado polínica de Butia, Euterpe, Geonoma, Syagrus e Thritrinax e implicares paleoecológicas de Arecaceae para o Rio Grande do Sul. IHERINGIA, Sér. Bot. 65: 3546. https://isb.emnuvens.com.br/iheringia/article/ view/79
BAUNI, V. & M. A. HOMBERG. 2015. Reserva Natural Campo San Juan. 1a ed. Fundación de Historia Natural Félix de Azara, Buenos Aires.
BHATTACHARYA, K., M. R. MAJUMDAR & S. GUPTA BHATTACHARYA. 2009. A Text Book of Palynology. New Central Book Agency (P) Ltd., Kolkata.
BOELCKE, O. 1992. Plantas Vasculares de la Argentina: nativas y exóticas. 2da ed. Hemisferio Sur, Buenos Aires.
CABRAL, E. L. & M. CASTRO. 2007. Palmeras Argentinas, Guía para el reconocimiento. L.O.L.A., Buenos Aires.
CABRERA, A. L. 1976. Regiones fitogeográficas argentinas. En: Kugler, W. F. (ed.), Enciclopedia argentina de agricultura y jardinería. Tomo 2. 2a. edición, Fascículo 1. pp. 1-85. Acme, Buenos Aires.
CACCAVARI, M. A. & E. DOME. 2001. Fabaceae-Mimosoideae. Tribu: Acacieae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. II: 6572. EUDENE, Corrientes.
CACCAVARI, M. A. & E. DOME. 2006. Fabaceae-Mimosoideae. Tribu: Mimoseae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.),
Flora Polínica del Nordeste Argentino, vol. III: 5569. EUDENE, Corrientes.
CARNEVALLI, R. 1994. Fitogeografía de la Provincia de Corrientes. LITOCOLOR, Asunción.
CECOTTI ÁLVAREZ, M. D., M. E. GARCÍA, N. J. F. REYES y A. C. SLANIS. 2017. Morfología polínica de las especies de Ludwigia (Onagraceae, Ludwigioideae) del Noroeste de Argentina. Lilloa 54: 29-40.
CONTRERAS-DUARTE, A. R., R. G. BOGOTÁ-ÁNGEL & L. C. JIMÉNEZ-BULLA. 2006. Atlas de las Esporas de Pteridófitos de Chipaque (Cundinamarca, Colombia). Caldasia 28: 327-357. CUADRADO, G. A. 1998a. Polygalaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. I: 81-88. EUDENE. Corrientes.
CUADRADO, G. A. 1998b. Sapotaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. I: 111117. EUDENE. Corrientes.
CUADRADO, G. A. 1998c. Polygalaceae. In: PIRE, S. M. , L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. I: 81-88. EUDENE, Corrientes.
CUADRADO, G. A. & J. J. NEIFF. 1993. Palynology of embalsados in distrophic lakes in northeastern of Argentina. Revista Brasil. Biol. 53: 443-451. CURTIS, H., N. S. BARNES, A. SCHNEK & G. FLORES. 2001. Biología. 6a. ed. en español. Panamericana, Buenos Aires.
DELLA,A. P. & J. PRADO. 2020. Jamesonia (Pteridaceae) in Brazil. Biota. Neotrop. 20: e20200986. https://doi. org/10.1590/1676-0611-BN-2020-0986 DELLA, A. P., J. PRADO & R. Y. HIRAI. Jamesonia. In: Flora do Brasil [online]. Available in: http://reflora. jbrj.gov.br/reflora/floradobrasil/FB134680 DÍEZ DAPENA, M. J., S. TALAVERA LOZANO y P. GARCÍA MURILLO. 1988. Contributions to the palynology of hydrophytic, non-entomophilous angiosperms. 1. Studies with LM and SEM. Candollea 43: 147-158.
ESKUCHE, U. G. 2004. La vegetación de la vega del rio Paraná medio superior, Argentina. Folia Bot. Geobot. Correntesiana 17:1-60.
FAEGRI, K. & D. IVERSEN. 1989. Texbook of pollen analysis. 4 ed. New John Willey & Sons, Chichester. FERNÁNDEZ, I. 1987. Contribución al conocimiento palinológico de Cyperaceae. Acta Bot. Malac. 12: 173-181.
FERNANDEZ PACELLA, L. 2013. Palinología del Cuaternario en sedimentos lacustres del Noroeste del Iberá, Corrientes, Argentina. Ph.D. Thesis. Universidad Nacional del Nordeste, Argentina. FERNANDEZ PACELLA, L. 2014. Morfología polínica de especies del género Senna (Fabaceae) del sureste del Iberá, Corrientes, Argentina. Rev. Biol. Trop. 62: 769-782.
FERNANDEZ PACELLA, L. 2015. Registro fósil y distribución de Anadenathera en Argentina desde el Mioceno hasta la actualidad. Estud. Geol. 71: e031. http://dx.doi.org/10.3989/egeol.41834.343 FERNANDEZ PACELLA, L. 2018. Estudio palinológico de un perfil sedimentario del Holoceno Medio-Tardío, oeste del Iberá, Corrientes, Argentina. Rev. Mex. Cienc. Geol. 35: 93-101. https://doi.org/10.22201/cgeo.20072902e.2018.E531 FERNANDEZ PACELLA, L. & O. CANTEROS. 2014. Poaceae. Tribus: Andropogoneae, Bromeae, Cynodonteae, Eragrostidae, Olyreae y Oryzeae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. IV: 88-94. EUDENE, Corrientes. FERNANDEZ PACELLA, L. & M. B. LARA. 2019. Paleoenvironmental Interpretation of the mid-late Holocene of Corrientes province, Argentina. Nordic J. Bot. 2019: e02252. http://dx.doi.org/10.1111/njb.02252 FERNANDEZ PACELLA, L., S. S. GARRALLA & L. M. ANZÓTEGUI. 2011. Cambios de la vegetación durante el Holoceno en la región Norte del Iberá. Provincia de Corrientes, Argentina. Rev. Biol. Trop. 59: 103-112.
FERNANDEZ PACELLA, L., L. M. ANZÓTEGUI & Y. HORN. 2014a. Fabaceae-Caesalpinioideae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. IV: 61-77. EUDENE, Corrientes. FERNANDEZ PACELLA, L., L. M. ANZÓTEGUI & L. R. MAUTINO. 2014b. Fabaceae-Mimosoideae. Tribu: Mimoseae-Prosopis In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. IV: 88-94. EUDENE, Corrientes.
FLORA DO BRASIL [online]. Available in: http:// floradobrasil.jbrj.gov.br/ [Access: 25 October 2021]. FONTANA, J. L. 2018. La Vegetación del Nordeste Argentino. 1. Las Comunidades vegetales del Noroeste de Corrientes y del Este de Chaco. Publicaciones Didácticas de la Cátedra de Ecología Vegetal. Vol. 3. UNNE, Corrientes.
FUERTES, E. & M. RODRÍGUEZ. 2009. Estudio sobre la morfología y germinación de las esporas de tres species de Sphagnum (Murci, Sphagnaceae). Bot. Complut. 33: 29-35. https://revistas.ucm.es/index. php/BOCM/article/view/BOCM0909110029A GARCÍA, A. V, H. S. LEYES, R. B. MARTÍNEZ, Y. J. PÉREZ, J. M. PINEIRO, M. E. PRIETO & S. C. SCHALLER. 2013. Guía de la Vegetación de la Estación Biológica Corrientes. In: FONTANA, J. L. (ed.), Publicaciones Didácticas de la Cátedra de Ecología Vegetal, vol. I: 44-51. Facultad de Ciencias Exactas, Naturales y Agrimensura, Corrientes. GARRALLA, S. S. 1998. Estudio palinológico de una secuencia sedimentaria del Holoceno, norte de Santa Fe, Argentina. Polen 9: 17-27.
GARRALLA, S. S. & G. A. CUADRADO. 2001. Meliaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. II: 95-100. EUDENE, Corrientes.
GENTILI, C. & RIMOLDI, H. 1979. Mesopotamia. II Simposio de Geología Regional Argentina. Academia Nacional de Ciencias en Córdoba, Vol. I: pp 185-222. GÓMEZ-NOGUEZ, F., B. PÉREZ-GARCÍA, A. MENDOZA-RUÍZ & A. OROZCO-SEGOVIA. 2013. Flora Palinológica de los Helechos y Licofitas de Río Malila, Hidalgo, México. Bot. sci. 91: 135154. https://doi.org/10.17129/botsci.410 GRADSTEIN, S. R. 2018. Key to hornworts (Anthocerotophyta) of Colombia. Caldasia 40: 262-270.
https://dx.doi.org/10.1544 6/caldasiav40n2.71750 HERBST, R. & SANTA CRUZ, J. 1985. Mapa litoestratigráfico de la Provincia de Corrientes. Revista D'Orbignyana 2: 1-51.
HIRAI, R. Y. & J. PRADO. Pellaea. In: Flora do Brasil [online]. Available in: http://reflora.jbrj.gov.br/ reflora/floradobrasil/FB91954 [Access: 2 November 2021].
IRIONDO, M. 1994. Los climas cuaternarios de la región Pampeana. Museo Provincial de Ciencias Naturales "FlorentinoAmeghino" 4: 481.
IRIONDO, M. 2010. Geología del Cuaternario en Argentina. Editorial Moglia, Corrientes.
KREMP, G. W. 1965. Morphologic Encyclopedia of Palynology. The University Arizona Press, Tucson. LORSCHEITTER, M. L., A. R.ASHRAF, R. MACHADO BUENO & V. MOSBRUGGER. 1998. Pteridophyte spores of Rio Grande do Sul flora, Brazil. Part I. Palaeontographica 246: 1-113.
MALDONADO, P. & E. HOHNE. 2006. Atlas del Gran Chaco americano. 1a ed. Agencia Alemana de Cooperación Técnica, Buenos Aires.
MARKGRAF, V. & H. L. D'ANTON! 1978. Polen Flora of Argentina-Modern Spore and Pollen Types of Pteridophyta, Gymnospermae andAngiospermae. The University of Arizona Press, Tucson.
MEDEANIC, S., C. V. CORDAZZO & L. G. LIMA. 2008. Diversidade Polínica de Plantas em Dunas do Extremo Sul do Brasil. GRAVEL 6: 67-80. Available in: http://repositorio.furg.br/handle/171867
MEZA TORRES, E. I., E. R. DE LA SOTA & M. S. FERRUCCI. 2013. Sinopsis de los helechos y licofitos del Parque Nacional Mburucuyá (Corrientes, Argentina). Claves de especies. Bol. Soc. Argent. Bot. 48: 121-136.
MORBELLI, M. A. 1977. Esporas de las especies argentinas de Selaginella (Selaginellaceae-Pteridophyta). Obra Cent. Mus. La Plata 3: 121-150.
MORBELLI, M. A., J. R. ROWLEY & D. CLAUGHER. 2001. Spore wall structure in Selaginella (Lycophyta) species growing in Argentina. Bol. Soc. Argent. Bot. 36: 315-368.
MORBELLI, M. A., M. R. PIÑEIRO & G. E. GIUDICE. 2010. Spore morphology and wall ultrastructure of Hymenophyllaceae Link (Pteridophyta) from north-west Argentina. Grana 49: 37-46. http://dx.doi.org/10.1080/00173130903483663
MORELLO, J. H. & A. F. RODRÍGUEZ. 2009. El Chaco Sin Bosques. 1a. ed. Ed. Orientación Gráfica, Buenos Aires.
NEIFF, J. J. 1997. Ecología evolutiva del macrosistema Iberá (Corrientes, Argentina). Master Thesis. Universidad Nacional del Litoral, Argentina.
NEIFF, J. J. 2004. El Iberá... ¿en peligro? Fundación Vida Silvestre, Buenos Aires.
NILSON, S. & J. PRAGLOWSKI. 1992. Erdtman's handook of palynology. Munksgaard International Publishers, Copenhagen.
OLIVEIRA, P. P. & F. RIBEIRO DO SANTOS. 2014. Prospecqao palinológica em méis da Bahia. Mídia Editora, Bahia.
ORFEO, O. 2005. Historia Geológica del Iberá, provincia de Corrientes, como escenario de biodiversidad. INSUGEO Miscelánea 14: 71-78.
ORFEO, O. & J. J. NEIFF. 2008. Esteros del Iberá: un enorme laboratorio a cielo abierto. Sitios de interés geológico de la República Argentina 46: 415-425.
PALDAT, A palynological database [online]. Available in: (2000 onwards, www.paldat.org) [Access: 5 November 2021].
PEÑALOZA-BOJACÁ, G. F., B. A., OLIVEIRA, C. A. T. ARAUJO, L. B. FANTECELLE, J. C. VILLARREAL & A. S. MACIEL-SILVA. Anthocerotaceae. In: Flora do Brasil [online]. Available in: http://reflora.jbrj.gov.br/reflora/ floradobrasil/FB97153 [Access: 3 April 2021] PÉREZ-JIMÉNEZ, J. C., F. ESLAVA-SILVA, K. JIMÉNEZ-DURÁN, F. GÓMEZ-NOGUEZ & M. E. MUÑIZ-DIAZ DE LEÓN. 2020. Estudio Palinológico de los helechos y licofitas de la Zona Núcleo Poniente de la Reserva Ecológica del Pedregal de San Ángel, Ciudad de México, México. Bot. Sci. 98: 517-532. https://doi.org/10.17129/botsci.2424 PIRE, S. M., L. M. ANZÓTEGUI, L. R. MAUTINO & S. S. GARRALLA. 2006. Acanthaceae. In: PIRE, S. M., L. M. ANZÓTEGUI & G. A. CUADRADO (eds.), Flora Polínica del Nordeste Argentino, vol. III: 15-38. EUDENE, Corrientes.
PPG I. 2016. The Pteridophyte Phylogeny Group. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 54: 563-603. PRIETO, A. R. & M. E. QUATTROCCHIO. 1993. Briófitas y Pteridófitas en sedimentos del Holoceno en la provincia de Buenos Aires, Argentina. An. Asoc. Palinol. Leng. Esp. 6: 17-37.
PUNT, W., P. P. HOEN, S. BLACKMORE, S. NILSSON & A. LE THOMAS. 2007. Glossary of pollen and spore terminology. Rev. Palaeobot. Palynol. 143: 1-81.
https://doi.org/10.1016/j.revpalbo.2006.06.008 RAMOS, V. 1999. Las provincias geológicas del territorio argentino. Instituto de Geología y Recursos Minerales. 29: 41-96.
RAVEN, P. H., R. F. EVERT & S. E. EICHHON. 1991.
Biología de las Plantas. T. I-II. Reverté, Barcelona. RINCÓN BARON, E. J., C. H. ROLLERI, L. M. PASSARELLI, S. ESPINOSA MATÍAS & A. M. TORRES. 2014. Esporogénesis, esporodermo y ornamentación de esporas maduras en Lycopodiaceae. Rev. Biol. Trop. 62: 1161-1195. SÁENZ LAÍN, C. 2004. Glosario de términos palinológicos. Lazaroa 25: 93-112.
SALGADO, C. R., 2006. Flora melífera de la provincia de Chaco. PROSAP y Ministerio de Producción del Chaco, Resistencia.
SERSIC, A. N. 1983. Ontogenia del esporangio y esporogénesis en Lycopodium saururus (Lycopodiales). Bol. Soc. Argent. Bot. 22: 205220.
TONELLO, M. S. & A. R. PRIETO. 2010: Tendencias climáticas para los pastizales pampeanos durante el Pleistoceno tardío-Holoceno: estimaciones cuantitativas basadas en secuencias polínicas fósiles. Ameghiniana 7: 501-514. https://www.ameghiniana. org.ar/index.php/ameghiniana/article/view/279 TRIGO, M. & I. FERNÁNDEZ. 1995. Contribución al estudio polínico de especies ornamentales con interés alergógeno en cultivadas en Málaga: Monocotiledóneas. Acta Bot. Malac. 20: 61-70. WINDISCH, P.G., C. G. V. RAMOS & B. OELLGAARD. Lycopodiaceae. In: Flora do Brasil [online].
Available in: http://floradobrasil.jbrj.gov.br/jabot/ floradobrasil/FB128519 [Access: 19 August 2021] YBERT, J. P., R. SCHEEL-YBERT & M. CARVALHO. 2016. Graos de pólen de plantas vasculares Dicotiledóneas do Estado do Rio de Janeiro, Brasil. Vol. I. Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro.
YBERT, J. P., M. CARVALHO & R. SCHEEL-YBERT. 2018. Graos de pólen de plantas vasculares do Estado do Rio de Janeiro, Brasil. Vol. IV. Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro.














