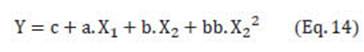Introduction
Stevia rebaudiana Bertoni, commonly called stevia, is a rich source of the natural sweeteners named steviol glycosides (SGs). Nowadays, stevia sweeteners are used in many countries in food formulations and for different pharmaceutical purposes. The percentage of SGs in stevia leaves varies from 4% to 20%, depending on the genotype, the phenotype and the agronomic practices (Kolb et al., 2001; Brandle and Telmer, 2007; Geuns, 2003). The main SGs in leaves are Stevioside, Stv (1 - 10%) and Rebaudioside A, RbA (2 - 13%); other SGs are normally found in smaller amounts (Morita et al., 2011; Wang et al., 2020; Pfister and Sehgal, 2012). Stv and RbA are largely responsible for the sweetness in stevia leaves; these SGs determine the quality of stevia for production process (Jonnala et al., 2006; Kinghorm et al., 2001).
The method to obtain SGs from stevia, in general involves pretreatments, extraction, purifications and crystallizations (Chhaya et al. 2012; Chabot and Beaulieu, 2012). The first step to obtain SGs is traditionally a discontinuous solid-liquid extraction (maceration or decoction) of pre-treated leaves (Abelyan et al., 2006; Chabot and Beaulieu, 2012; Jonnala et al., 2006; Kienle, 1992). SGs are extracted from leaves with water or alcohols (Barba et al., 2015; Ameer et al., 2017). New extraction techniques are being used such as: microwave-assisted extraction, pressurized liquid extraction, supercritical-fluid extraction, ultrasound-assisted extraction, high pressure assisted extraction and enzyme-assisted extraction (Bursać-Kovačević et al., 2018; Xu et al., 2019). Selection operation technique and operation condition affect directly the quality of stevia extract (Chabot and Beaulieu, 2012). The product in the extraction step (crude extract) is a colored and dense mixture containing SGs, among other components of leaves (Chabot and Beaulieu, 2012). Crude extract is normally subject to a multi-step purification to obtain individual components (Kienle, 1992; Payzant et al., 1999; Wang et al., 2020): Stv, RbA, Rebaudioside D, Rebaudioside C and SGs mixtures.
There are some desirable requirements for the industrial production of SGs: high processing capacity, high yields, high concentrations and high purity. In a previous work, Persinons (1973) used a percolator for the recovery of Stv from pre-treated stevia leaves. More recently Fromigoni et al. (2018) used a column to treat stevia leaves with ethanoic solvent. In another study, Ameer et al. (2017) optimized the ethanol-water solvent for the SGs recovery. Raspe et al. (2021) applied sonication for increase the recovery of SGs with ethanol-water solvents and Ciotta et al. (2022) used a semicontinuous extraction with boiling water followed by microfiltration to obtain purified stevia extracts used as sweetener.
In South American countries, there are diverse commercially-available stevia products since the approval of SGs as food additives (CAA, 2011; Celaya et al., 2021; JECFA/69, 2008). Despite this, in Argentina there is currently no large-scale SGs industrial production. For this reason, there is a strong need to explore the possibilities inherent in stevia cultivation and SGs industrial production in the country.
In the present study, we aim to optimize the recovery of Stv and RbA form stevia leaves, in a semi-continuous process in column percolation by using ethanol-water as solvent, while working at moderate temperatures (from 30 to 70°C) in the extraction process. The kinetic for the recovery of Stv and RbA was established in the best conditions for the extraction.
Materials and methods
Chemicals and plant material
HPLC grade solvents were supplied by Merck (Darmstadt, Germany). Cicarelli (Reagents S.A., San Lorenzo, Argentina) supplied ethanol. The distilled water used for the extraction was deionized in a Milli-Q system. Stv (purity of 95.0%) and RbA (purity of 99.5%) were obtained in-house by re-crystallizations in methanol and ethanol, as determined by a chromatographic analysis (Celaya et al., 2021). The binary solvents employed in the percolation study were prepared by mixing ethanol and water in adequate proportion to obtain dissolutions with density according to reported values (Celaya et al. 2016; Poling et al., 2008). All other chemicals were analytical grade.
Stevia was harvested in the experimental farm when the flowers were opened (0-5% open flower), in Posadas (Departamento Capital, Argentina). Samples were collected after sun-drying for 4 days. Stevia leaves were ground and sieved to obtain 70% of particle size of 5-40 mesh and 30% of particle size > 40 mesh (Norma IRAM 20514/2007). The SGs contents were quantified as described below. The moisture content was determined by drying samples (1-3 g) at 102 ± 2 °C until it reached constant weight. Stevia leaves pre-dried and grinded contained the following SGs: RbA 10.1 ± 0.6% and Stv 2.4 ± 0.1%. The lot contained the following amount of moisture: 7.9 ± 3.1%.
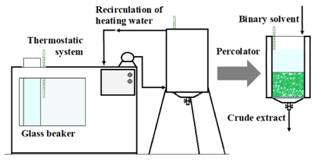
Sample extraction with the percolator
The solid/liquid extraction of SGs was performed by means of a lab-scale percolator. The percolator consists in a cylindrical vessel in which the solids rest on a cotton bed on a perforated support (Fig. 1). The temperature of percolation was controlled by a thermostatic system with recirculation of heating water (Shott Gerate, model CTI 150, USA). The extraction temperature was measured by three thermometers. The solvent extraction was adjusted for the assay by immersing a glass beaker (containing the binary solvent) into the thermostatic system (Fig. 1).
The stevia weight and percolation flowrate were determined in preliminary experiments. For each test, 200 g of dried and grinded leaves were packed and impregnated with 1700 mL of solvent. After impregnation (6 min), the solvent was eluted by using gravity and 10 fractions of 100 mL were obtained. The total percolation time used was 60 min; the flowrate used was 16.7 mL/min. After extraction, the fractions were rapidly cooled to room temperature and filtered. Afterward, the fractions were mixed and a total extract of 1000 mL was used for the optimization.
Soxhlet extraction
For comparison purposes, multiple-batch extractions were carried out employing a soxhlet system (Rouhani, 2019). The solvents used were: water, ethanol 96° and ethanol. Ground stevia leaves were pre-dried at 60°C for 2.0 h; then 20 g of stevia were placed in an extraction thimble. The thimble was placed in the soxhlet extractor. The plant material was extracted using 500 mL of solvent for 10 h. The residual solvent was removed by evaporation at 60°C up to constant weight. Extracted materials and extracts were used for analytical purposes.
HPLC quantification of SGs in leaves and extracts
The quantification of SGs in leaves and extracts was performed with a high-performance liquid chromatography diode array detector (HPLC-DAD) by using the external standard method proposed by Kolb et al. (2001). The analyses were performed on a Prominence LC-20AT chromatograph (Shimadzu Corporation, Kyoto, Japan). The operating conditions were as follows: the column was Agilent Zorbax NH2 (25.0 × 0.46 cm; 5 µm, particle size; Waters, Milford, MA), the UV detector was set at 210 nm, samples were run at room temperature, the mobile phase was acetonitrile:water 80:20 (v/v), and pH was 5.
For the calibration curve, stock solutions of 0.2-1.0 g/L of standard compounds (Stv and RbA) in ethanol-water 70:30 (w/w) were prepared. The parameters for external calibration curves were obtained by fitting experimental data through linear regression from replicate injections of standard solutions. The compounds in each sample were identified by comparing their retention times with those of the standards.
Factorial design
The solid/liquid extractions of SGs were carried out under the experimental conditions defined in a factorial design 3x5 (Table S1). The factorial design 3x5 was used to study the effect of temperature, X 1 (30°C, 50°C and 70°C) and ethanol percentage, 𝑋 2 (0%, 30%, 50%, 70%, 100%), on the responses: purity of extracts, retention of solvents in solid waste and RbA/Stv ratio. The statistical software Statgraphics Plus 5.1 software (Manugistics, Rockville, MD, USA) was used to carry out this study.
Optimization of concentration and recovery
Temperature ( 𝑋 1 ) and ethanol percentage ( 𝑋 2 ) were optimized using response surface method. The dependent variables were: concentration (amount of SGs and solids extracted) and recovery (Stv and RbA). The total amount of solids extracted (TSE) was measured after drying 10 mL of crude extract at 102 ± 2 °C until it reached constant weight on a tared steel plate (Celaya et al., 2021).
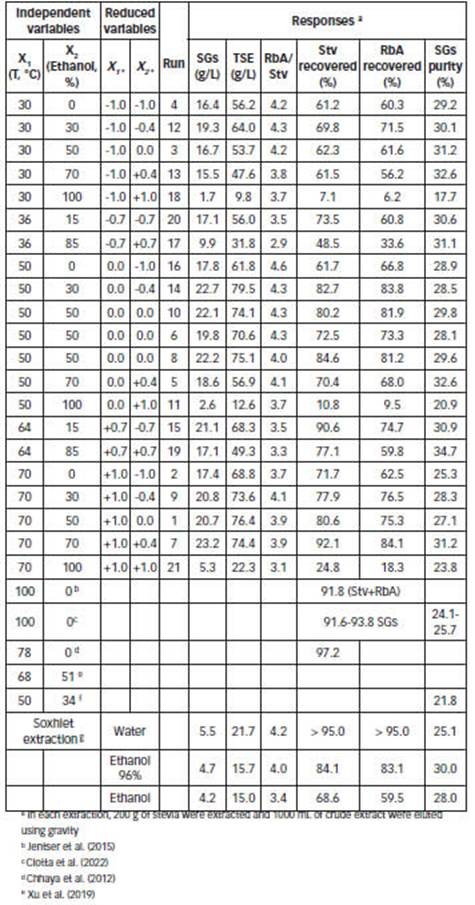
The experimental ranges selected for the independent variables were: temperature (from 30 to 70°C) and percentage of ethanol in the binary solvent (from 0 to 100%). The experimental conditions and responses for conducting the experiments were analyzed following a pre-defined composite design with 19 experiments summarized in Table 1. Two replicates of the central point were conducted.
Equations
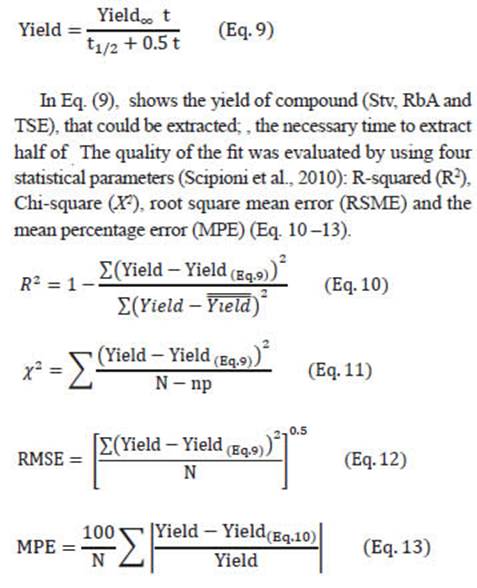
The variables determined in the extracts were: concentration (g/L), recovery of sweeteners (%, dried basis), purity (% of crude extracts) and yield (on a dried basis).
The concentration in the extract was determined as:
In Eq. (8), Y is the response, concentration and recovery (SGs and TSE); c, a, aa, b, bb, ab are the coefficients of the equation. Significant effects (P < 0.05) were defined by using a standardized Pareto chart dropping coefficients without statistical significance. Models established were used to find the optimal mixture compositions with desirable responses (i.e., maximum concentration, maximum recovery) using the “Response Optimizer” (evaluated in Statgraphics Plus 5.1).
Extraction kinetics
SGs extraction kinetics were undertaken in the stevia percolator under selected conditions for recovery and purity.
When performing the stevia percolation process in these selected conditions 10 fractions of 100 mL were obtained. The total percolation time used was 60 min. The fractions were rapidly cooled to room temperature and filtered. The 10 fractions (100 mL in 6 min each one) were used for the kinetic study. Extractions were carried out in triplicate. The experimental data was adapted to the model of Pilosof et al. (1985):
Results and discussion
Optimization of concentration and recovery
Out of the dependent variables analyzed here, only the behavior of concentration (and recovery) can be described by the polynomial Eq. (8), proposed by the software Statgraphics Plus 5.1 by using the standardized Pareto chart dropping coefficients without statistical significance. The final equation that predicts concentrations (and recovery) is:
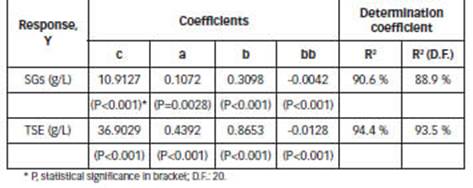
The response surface plots that predict the concentration of extracts (SGs and TSE), for extraction volume of 1000 mL (Table 1) are shown in Fig A1 (Appendix). Coefficients c, a, b, bb are shown in Table 2.
The extraction efficiency could be influenced by multiple parameters, such as time, temperature and solvent polarity and their effects may be either independent or interactive (Jentzer et al., 2015). The responses show linear temperature dependence and a quadratic dependence of ethanol percentage (Table 2, Fig.A1). Different results were obtained in another study (Raspe et al., 2021), investigating the influence of temperature, and binary mixtures of water and ethanol as solvent.
In the soxhlet extraction, 20 g of stevia were extracted with 500 mL of solvent
Temperatures from 50°C to 78°C were selected in other works, to obtain higher SGs values with different extraction process (Afandi et al., 2013; Chhaya et al., 2012; Raspe et al., 2021; Rouhani, 2019; Xu et al., 2019). Higher temperatures lead to better SGs solubility, faster diffusion rates, lower solvent viscosities and weakening of the solute-matrix interactions (Ciotta et al., 2022; Jentzer et al., 2015). However, when working with alcohol-water mixtures, higher temperatures may cause preferential vaporization and ethanol loss (Singh, 2008). The high temperature used here is below the boiling point of ethanol, avoiding high loss of preferential evaporation of the organic solvent during the extraction process.
The selection of the best solvent to extract the phytochemicals from plant materials is essential (Rouhani, 2019). The effects of ethanol percentage on the SGs recovery indicates that binary solvents resulted in a higher concentration of SGs in crude extracts (Fig. 2 and Fig. A1). These results are in accordance with previous findings (Celaya et al., 2016; Raspe et al., 2021; Xu et al., 2019). In general, binary solvents effectively promote the solubilization of SGs, and this behavior is favored by the presence of other SGs and biomolecules. Previously, Afandi et al. (2013) obtained a different result in the recovery of SGs with the best recovery in pure alcohol.
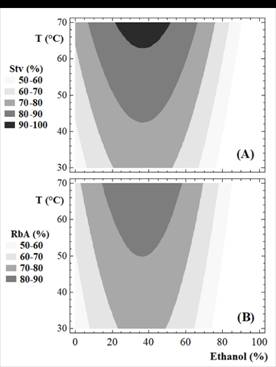
The effect of temperature and ethanol percentage on the Stv and RbA recovery is shown in Fig. 2. According to the results obtained (Fig. 2.A and Fig. 2.B), binary solvents are superior to those found in water and ethanol up to 70 °C, since it allows for a more rapid recovery of Stv and RbA at moderate temperatures.
Recovery of Stv and RbA were maximized and the optimized process conditions were: temperature: 70°C and ethanol: 35%. When the solvent to leaf ratio was equivalent to 5:1 (1000 mL/ 200 g), the predicted recoveries were 93.0% and 87.5% for Stv and RbA, respectively.
Effects of percolation conditions on purity
In order to conduct research on the effects of percolation conditions on the purity, a 3x5 factorial design was used (Table A1). The effect of ethanol percentage on the SGs purity is shown in Fig. 3 (and Table 1). It indicates that binary solvents with high percentage of ethanol result in higher purity of crude extracts. On the other hand, over the temperature range (between 30-70 °C), temperature had no effect on the purity (P > 0.05; CL 95%).
Selection of the solvent for a percolation process depends on the solubility of the desired components in the solvent (Singh, 2008). The impurities present in stevia extracts are components co-extracted with SGs from the leaves (Žlabur et al., 2015; Xu et al., 2019); the impurities include saccharides and crude fiber, chlorophylls, phenolic compounds, resins, phytosterols and triterpenes, proteins, organic acids, fatty acids, gums and colloids (Celaya et al., 2021; Chabot and Beaulieu, 2013).
For Ameer et al. (2017) and Xu et al. (2019), 75% and 59% of ethanol v/v (76% and 59% of ethanol w/w) was superior to other binary solvents for a maximum SGs yield in the extraction of Stv and RbA from stevia. Different was for Raspe et al. (2021), with the best result at 40% of ethanol v/v (34% w/w).
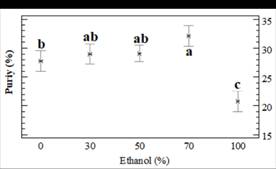
Figure 3: Dependence of purity (Eq. 5) with ethanol (%, w/w) in a binary solvent for SGs extraction (statistical significance, P 0.004; D.F. 14).
In order to obtain a better SGs recovery in the present study, the 35% (w/w) was optimal (Fig. 2.A and Fig. 2.B). On the other hand, a percentage of ethanol of 70% (w/w), similar to proposed previously (Ameer et al., 2017; Chabot and Beaulieu, 2012; Kolb et al., 2001), was appropriate for the recovery of an extract with the best purity. In the present study, the high purity was for 85% w/w of ethanol at 70°C (Table 1, Fig. 3), but the recovery with this binary solvent is very low (77.1% for Stv and 59.8% for RbA).
RbA/Stv ratio
The effect of the percolation conditions on the RbA/Stv ratio over the extracts was also been statistically analyzed by means of the 3x5 factorial design (Table A1). The RbA/Stv relations that appear in the extracts are summarized in Table 1. Stv and RbA show a similar behavior in the percolation, but there are some differences regarding the recoveries (Figure 2). Xu et al. (2019) obtained similar results previously, in the recovery of SGs for analytical purposes. RbA contains one more glycosyl group than Stv. RbA can form more hydrogen bonds with water or alcohols (Celaya et al., 2016). A higher RbA in relation to Stv was favored by the presence of a high percentage of water in the binary solvent (Figure 4.A). Temperature had an impact on this variation. Such differences could be reflected on the RbA/Stv ratio (Figure 4.B). Based on the results, in general lower temperatures and lower percentage of ethanol improve RbA/Stv ratio.
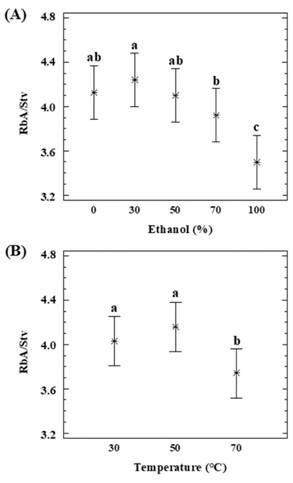
Figure 4: Dependence of RbA/Stv ratio with (A) a percentage of ethanol (P 0.0028, D.F. 14) and (B) temperature (P 0.0088; D.F. 14).
Soxhlet extraction
Soxhlet apparatus is a laboratory equipment that works at solvent boiling temperature (Takeuchi et al., 2009). The procedure was carried out in this study with ethanol, ethanol 96 and water. The obtained yields in these extractions are shown in Table 1. Previously, Rouhani (2019) obtained different results for the soxhlet extraction of Stv from stevia leaves. According to the results obtained here, polar solvents result in a higher RbA/Stv ratio and high recovery. The solvent ethanol 96% result in a higher SGs purity.
In other study, Ciotta et al. (2022) used semicontinuous percolation with boiling water as solvent to recover SGs from stevia leaves. In the study, Ciotta and co-workers used five extractive cycles to recover 91.64 of the SGs present in the leaves. Here the best recovery was for soxhlet extraction using boiling water as solvent (recovery > 95.0 %).
Extraction kinetics
The chosen conditions for the kinetic study were 35% of ethanol at 70°C (condition 1, fast recovery) and 70% of ethanol at 70°C (condition 2, best purity). SGs extraction kinetics were undertaken in the percolator under these selected conditions. The data of ten fractions of 100 mL of crude extract obtained in each condition (percolation extract, 1000 mL), were adapted to the model of Pilosof et al. (1985). This model was previously applied to study the release rate of RbA from matrices formed with agglutinants in yerba-mate powder (Scipioni et al., 2010). The results are in agreement with the recovery and purity previously estimated.
Figures 5.A and 5.B show the experimental and predicted yields obtained with the Pilosof model. The regression coefficients and statistical parameters (Eq. 9) are listed in Table 3. A good fitness was obtained in all cases; R2 values were high (>95%), and χ 2 , RSME and MPE were low.
According to the model, high values of t 1/2 indicate a low recovery rate of compounds. The highest value of 𝑡 1/2 was obtained for TSE with 70% of ethanol at 70°C (68.6 min). SGs showed the lowest 𝑡 1/2 values, in 35% of ethanol at 70°C, 27.9 and 28.9 min for Stv and RbA, respectively (Table 3).
For Raspe et al. (2021), the increase in the solvent/leaf ratio provided the highest yield of total solids and SGs, as the increase of ethanol in the solvent, while the temperature promoted an increase only in the of total solids. The result of kinetic behavior obtained here are in accordance with the results of Raspe ad coworkers from 54 min of percolation (Figures 5.A and 5.B).
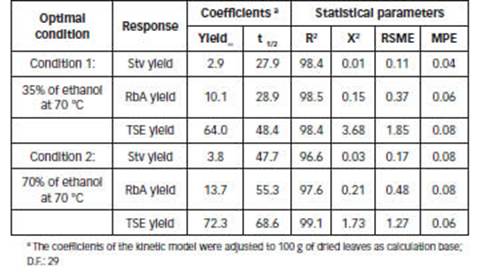
Table 3: Constants and statistical parameters of the kinetic model of Pilosof (Eq. 9) for optimal conditions.
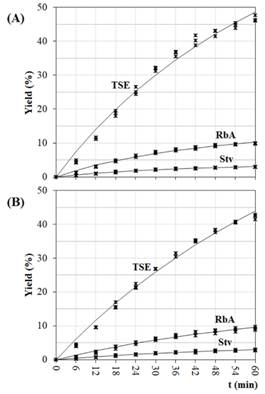
Figure 5: Extraction kinetics in the best conditions: (A) 35% of ethanol at 70 °C; (B) 70% of ethanol at 70 °C. Coefficients for the kinetic models (Eq. 9), are listed in Table 3.
Conclusions
In this study we aim to optimize the recovery of Stv and RbA in a stevia percolator by using mixtures of ethanol and water, while working at moderate temperatures in the extraction process. When using ethanol-water as a solvent for the percolation, the optimum extraction temperature is 70°C. Regarding the percentage of ethanol, the statistical analysis showed that the optimal extraction conditions, which gave the best SGs extraction kinetic is 35% of ethanol, but the highest purity (at least 31%) is obtained with 70% of ethanol. Based on the results, it is not possible to obtain the fast SGs recovery and high purity at the same time in the stevia percolation extraction with binary solvent.
The obtained results could be used industrially for the process of stevia leaves obtaining crude extracts with high purity or high yield.