Introduction
Thrips are small, highly thigmotactic insects. Females of the suborder Terebrantia introduce their eggs in petioles, stems, leaves, and fruits, and therefore rapid visual detection is extremely difficult 11,13. The “pear thrips” Taeniothrips inconsequens (Uzel) economi cally affects exports of stone fruit propagation materials. Then, certain requirements for the phytosanitary certification of shipments of apricot propagation material from Argentina to other countries such as Peru were required 18.
The pear thrips has a wide range of host plants, stone and pip fruit trees, as well as ornamental and forest plants 20. The common name of the species refers to its associ ation with the pear tree. However, most of its damage is in Acer saccharum Marshall in the northern United States and Canada 19,21,22.
Taeniothrips inconsequens is native to Europe and entered America through the north of the United States and Canada 9,15. In Argentina, it was cited on pear for the first time by Joan in 1921 10. Then, De Santis and co-workers in 1978 8 reported others host plants (almond, peach and apricot trees) and its geographic distribution (Buenos Aires, Mendoza and Neuquén provinces) in Argentina. In the last 20 years, the pear thrips has not been mentioned in studies involving the identification of thrips on fruit trees in Argentina 3,4,5,6,16,23. We have never seen this thrips species in Argentina and we believe that the pear thrips is not present in this country. This study was conducted to verify the presence of T. inconsequens in Argentina.
Materials and methods
Sampling and collecting thrips from fruit branches
The sampling was carried out between 2017 and 2019 during the flowering or sprouting stages of nine species of fruit trees in the six main producing provinces of stone and pip fruits in Argentina (Tables 1, page 111 and 2, page 112).
Table 1: Number of fruit orchards, ordered by provinces and counties, with their respec tive geographic coordinates, altitude ranges and sampling times.
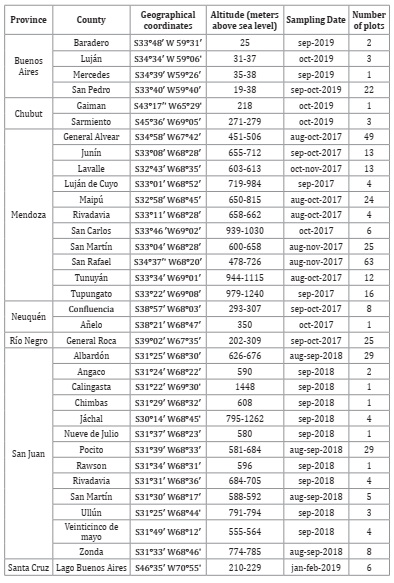
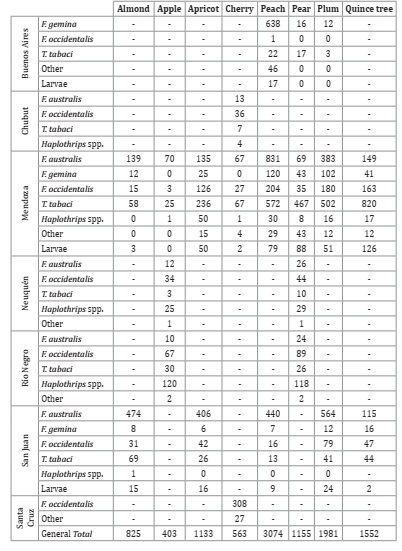
Table 2: Numbers of thrips collected by species and province on a total of 393 samples taken in Argentina.
The sampling was performed by manually shaking a branch over a 0.40 m x 0.30 m white tray. Thrips collected on the tray were transferred with a brush to vials with preser vative liquid (10% ethyl alcohol aqueous solution, 5% acetic acid and 0.1% Triton; Bhatti, J., personal communication). Collected thrips were placed in a 70% ethanol aqueous solution for their final conservation.
Thrips were collected using systematic U sampling. For every ten plants one was sampled. One branch at 1.5 m height per tree was selected for the sampling. Thrips were collected completing approximately a sample of 30 specimens per orchard. When the number of thrips was low, all the specimens were collected in a time period of 30 minutes.
Geographic coordinates were recorded with a G.P.S., dates and plant species and pheno logical stage were registered.
Thrips identification
Most of the adult specimens were identified under a stereoscopic microscope with an 80 x magnification. Some specimens of each species were mounted on microscopic slides following the Mound and Marullo technique 12 and identified by keys and descriptions 1,4,7,8,9,14 or by confrontation with previously identified material from the thrips collections of INTA Mendoza (EEA Mza INTA) and of the Museo de Ciencias Naturales de La Plata (MLP https:// www.museo.fcnym.unlp.edu.ar/ . All specimens collected were preserved in vials with 70% ethanol or microscopic slides at the Entomology Lab of the EEA Mendoza INTA.
Results
A total of 10,686 individuals of thrips from 393 samples of fruit branches from the main stone and pip fruit producing areas of Argentina were collected. Taeniothrips inconsequens (Figure 1A, page 114) was not found.
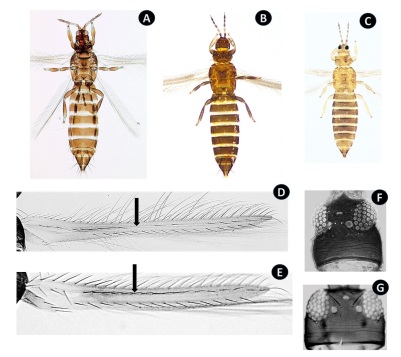
Adult female. A, T. incon sequens (image by Mound et al., 2018); B, F. austra lis; C, T. tabaci. Fore wing. D, T. inconsequens (image by Mound et al., 2018); E, F. australis (indicated with an arrow the first vein of the fore wing). Head, F, T. inconsequens; G, F. australis. Hembras adultas. A, T. inconsequens (imagen de Mound et al., 2018); B, F. australis; C, T. taba ci. Alas anteriores. D, T. inconsequens (imagen de Mound et al., 2018); E, F. australis (indicado con una flecha la prime ra vena del ala anterior). Cabezas, F, T. inconse quens; G, F. australis.
Figura 1: Comparación de Taeniothrips inconsequens con Frankliniella australis y Thrips tabaci.
Ninety percent of the collected thrips corresponded to four species: 37% Frankliniella australis (Figure 1B, G y E, page 114), 29% Thrips tabaci Lindeman (Figure 1C, page 114), 14% Frankliniella occidentalis (Pergande), and 10% Frankliniella gemina Bagnall. The remaining 10% consisted of 2% larvae and 8% adults of the following species, Aneristothrips rostratus De Santis, Frankliniella frumenti Moulton, Frankliniella schultzei (Trybom), Frankliniella inesae de Borbón & Zamar, Frankliniella juan carlosi de Borbón & Zamar, Frankliniella spp, Leptothrips mali (Fitch), Aeolothrips fasci atipennis Blanchard, Arorathrips texanus (Andre), Tenothrips frici (Uzel), Haplothrips spp, Haplothrips fiebrigi Priesner, Haplothrips trellesi Moulton, Thrips australis (Bagnall), Karn yothrips spp., Caliothrips phaseoli (Hood).
Regarding the distribution of the species in the studied areas, F. gemina was dominant in Buenos Aires, T. tabaci in Mendoza, Haplothrips spp. in Río Negro and Neuquén, F. occi dentalis in Chubut and Santa Cruz, and F. australis Morgan in San Juan (Table 2, page 112). In relation to the host plants, F. australis was dominant in almond and peach trees, while T. tabaci was dominant in pear and quince trees.
Discussion
In Joan's work (1991), samples of pear flowers were collected only in the Ituzaingó county in the province of Buenos Aires. The characters used by Joan to describe and illustrate T. incon sequence are shared by many other thrips species or are imprecise and diagnostic features of pear thrips are not specified. Furthermore, the author does not indicate where the examined material was deposited in order to study it. The citation about the pear thrips for the province of Buenos Aires 10 can be attributed to a misidentification. This error could be due to the limited knowledge about thrips taxonomy at that time, the limited availability of information and the non-corroboration of the species determination by a thrips specialist.
Joan (1991) describes a species of brown colour, with eight-segmented antennae, and a head that is wider than long. Although the first two features are correct, the last one is erroneous. The head of T. inconsequens is longer than wide 9,14. Also in Joan’s paper, the illustrations are imprecise, the larva drawing shows more information, with respiration spiracles in tergites II and VIII and numerous tooth-shaped processes in tergite IX. The distribution of the setae in the tergites and the shape of the antenna are those observed in species of the family Thripidae. If the illustration of Joan's larva is correct, the pear thrips is ruled out because the tooth-shaped processes of tergite IX are medium size and numerous, while in T. inconsequens they are large and scarce (three pairs) 17.
Other common species on fruit trees in Buenos Aires could also motivate erroneous identifications. Among the most common brown species that can be found is T. tabaci. This species differs from T. incosequens in that it has uniformly pale forewings and the head is wider than long, and it does not show constriction (Figure 1A-G, page 114). Another frequent, brown-coloured species is Frankliniella schultzei. It is distinguished from T. inconsequens because adults have a pair of long setae on the anterior margin and another on the anterior angles of the pronotum. The forewings are uniformly pale, the head is wider than long and without posterior constriction to the eyes.
Pear thrips is also cited by De Santis and co-workers (1978). These authors provide data on the host species (pear, almond, peach, and apricot) and their geographic distribution for Argentina (Buenos Aires, Mendoza and Río Negro). However, they do not indicate where this information was obtained from. On the other hand, no slides labelled as T. inconsequens with specimens collected in Argentina are at the Museo de la Plata.
The citations of T. inconsequens in Mendoza and Río Negro 2,8 can be attributed to mistakes over mistakes which originated in erroneous identifications made by non-spe cialists and later mentioned in successive citations. Frankliniella australis (Figure 1B) resembles T. inconsequens (Figure 1A) in its body and antenna coloration. To distinguish them, it is necessary to observe in detail the pronotum setae, the venation of the forewings and the shape of the head, characteristics that can be visualized under a stereomicroscope at 80 x magnification. Taeniothrips inconsequens does not have long setae on the margin and anterior angles of the pronotum, it has the first vein with setae arranged discontinuously (Figure 1D) and the head has a constriction posterior to the eyes giving the appearance of swollen genae; (Figure 1F) while F. australis, (Figure 1E) has a pair of long setae in the anterior margin and another in the anterior angles of the pronotum, the first vein has a continuous row of setae; the margins of the genae are almost parallel, with no obvious constriction behind the eyes (Figure 1G). These species also have other important differences. T. incon sequens has only two pairs of ocellar setae and has a small spur on the tarsal apex of the forelegs (a character that differentiates it from other species of the genus Taeniothrips), and it does not have ctenidia placed anterior to the spiracles of respiration. While F. australis has three pairs of ocellar setae, a spur on the tarsi of the fore legs is not present, and tergite VIII has a pair of ctenidia located anterior to the spiracles of respiration.
Considering that no slides of specimens collected in Argentina of T. inconsequens were found at the Museo de La Plata, in De Santis and co-workers’ paper (1978), it is considered that these authors make references to other articles that cite the species but no to identifi cations made by themselves.
In our research, the presence of some species of thrips in fruit trees was accidental due to the fact that they have other host plants. Thus, Arorathrips texanus and Frankliniella frumenti live on grasses, while Tenothrips frici is common on asteraceae, Thrips australis on eucalyptus, Frankliniella inesae on asteraceae, mainly of the Baccharis genus. Some species found in low frequency, which are predators or potential predators, were Leptothrips mali, Aeolothrips faciatipennis and Karnyothrips spp. Other species, such as F. schultzei and Calio thrips phaseoli, may have alternative fruit hosts.
Conclusions
It is possible, based on our findings, that the citation of T. inconsequens for Argentina is a misidentification of another species. The pear thrips was not found during blooming and or sprouting of nine kinds of stone and pip fruit in Argentina's primary producing regions. In addition, no slides of T. inconsequens from Argentina were found at the museum of La Plata. The pear thrips should be excluded from the Argentine fauna and considered as a quarantine species to prevent its presence.














