Introduction
Feeding corn silage to dairy cows is a growing practice in the Mexican tropics. Maximizing nutrient bioavailability of corn silage would represent important economic and productive advantages. Specifically, enhancing cell wall degradability might improve profits while reducing both solid excretion and methane production, and potentially reducing grain level in feed up to 3% (40).
Plant cell walls are a major source of energy for ruminants. However, only less than 50% are readily digested and utilized by the ruminant host (3). In fact, the low degradability of cell wall fractions (cellulose, hemicellulose, pectin and lignin) is a key factor limiting its use by rumen microorganisms. In addition to corn variety, crop maturity, kernel processing and particle cut length (12), another alternative to improve fiber degradability of corn silage is to increase cutting height at harvest (9) improving both forage quality and milk production (9,12,21).
Lactic acid bacteria (LAB) inoculants and exogenous fibrolytic enzymes (EFE) have become popular as silage additives intended to improve silage nutritional value (5,17,26). LAB improve fermentation characteristics by promoting faster pH decline, avoiding both proteolysis and excessive fermentation of water-soluble carbohydrates (WSC) (25). In addition, LAB also improve the lactic acid-acetic acid ratio and promote low ammonia nitrogen content (38). Furthermore, dry matter loss is reduced by 35%, while intake, digestibility, weight gain, and milk production are also improved (38). Exogenous fibrolytic enzymes promote cellulose and hemicellulose hydrolysis, therefore improving cell wall degradation while releasing WSC, which undergoes subsequent fermentation by LAB (15,33). Additionally, the enzymatic additives improve fermentation by stimulating the production of acids and lowering pH and ammonia nitrogen in the silage (38). When EFE are added to forage, parameters like digestibility, dry matter intake and milk production, usually increase (38). However, data on how EFE affects digestibility of DM and NDF have been mostly inconsistent (17,38).
Among other factors, LAB efficacy depends on WSC content of the forage to be ensiled, while EFE extent of action is related to both NDF content and chemical composition. It is hypothesized that changing the grain-to-stover ratio through cutting height might promote the synergistic effect between LAB and EFE on silage digestibility. This study aimed to assess the single and combined effects of LAB and/or EFE on chemical composition and in vitro digestibility of the DM and NDF in corn silage when corn is harvested at different cutting heights.
Materials and methods
Corn was planted in Jose Azueta, Veracruz, Mexico; at 18°05’50” N and 95°39’35’’ W, and 16 m above sea level. Local climate is warm and subhumid with summer rains (Aw1). Maximum, mean and minimum temperatures are 31.8, 26.3 and 20.8°C, with an annual rainfall of 1601 mm (16). The most common soil types are gleysol (39%) and phaeozem (39%).
Hybrid corn DK-357 (DEKALB®) was established on November 29 of 2017, and grown under rainfed conditions. Sowing density was 75,000 seeds ha-1, at 80 cm between rows. Fertilization consisted of 175 kg ha-1 of diammonium phosphate (DAP, 18-46-00) during sowing.
Treatments and silages
Corn was harvested when dry matter content approached 35%, around day 103 after planting. On the plot, ten out of 108 rows were randomly selected and three 10 m long sections were sampled at 20, 150 and 280 m from the end row. On every sampling, plant number and height, stem diameter and node number were recorded. Then, six random plants were cut at 12, 25 or 42 cm cutting heights (CH). A total of 60 plants were harvested for every CH. Plants were then transported to the Laboratory of the Universidad del Papaloapan, in Loma Bonita, Oaxaca.
Fifty-five out of the 60 plants, were assigned to four treatments: without additive (C, control) and with additives (AD) Fibrozyme® (F), Sil-All® (L), and with Fibrozyme plus Sil-All (FL). According to manufacturers, Fibrozyme® (Alltech Inc., Nicholasville, KY, USA) is a fermentation extract of Aspergillus niger and Trichoderma viride with 31.0 and 43.4 IU of xylanase and cellulase activity, respectively (11). Sil-All® (Lallemand Specialties, Inc. Milwaukee, WI, USA) contains Lactobacillus plantarum, Pediococcus acidilactici, Enterococcus faecium, Lactobacillus salivarius at a minimum ratio of 2.1 x 1010 CFU g-1.
Plants were cut with a stationary gas chopper (Raiken® RKP-3000B) before filling three laboratory silos with their corresponding treatment (C12, C25, C42, F12, F25, F42, L12, L25, L42 or FL12, FL25, FL42). Laboratory silos with a capacity of 3.0 kg of fresh forage, were made from PVC tubes (15.7” high x 4” wide). Sil-All dosage was provided according to manufacturer’s specifications (10 mg kg-1 of fresh forage). Fibrozyme dosage was 60 mg kg-1 of the DM to be ensiled. Twenty-two milliliters of distilled water dissolved each additive, and then the solution was spread over the chopped forage. Treatment C consisted of 22 mL of distilled water spread over the forage. Then, laboratory silos were filled up and compacted manually, covered with a grow bag and sealed with brown tape. The 36 laboratory silos were weighted and kept at room temperature in the Laboratory of the Universidad del Papaloapan for 50 days.
Chemical analysis
After 50 d of ensiling, all laboratory silos were weighted and then opened. Considering each silo, pH was assessed in a 25 g sample mixed in 250 mL of distilled water and blended for 30 s at maximum speed. The solution was filtered through two cheesecloth layers before taking the pH readings with a HANNA® potentiometer (model pH 209, Instruments Inc. USA).
Partial dry matter content was determined with 500 g samples from each silo, oven dried at 60°C for 48 h and ground through a Wiley® mill to pass a 1 mm screen (10). Ground samples were used to estimate total dry matter (method # 930.15), ash (method # 942.05) and crude protein content (CP, method # 990.03), according to AOAC (2006). Furthermore, cell wall fractions of neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined. The NDF analysis was carried out using Na2SO3 and α-amylase. ADL analysis was run in a beaker by immersion in 72% H2SO4. Cell-wall fractions content was determined sequentially in the ANKOM200® fiber analyzer, using Ankom F57® filter bags, following the procedures proposed by the company. Additionally, non-fibrous carbohydrates (NFC) content was calculated using the equation: NFC (%) = 100 - [NDF% + CP% + ether extract % + ash%], where NDF was not corrected for CP or ash, and ether extract was considered 3.2% for all silages (28).
In vitro digestibility of DM and NDF
In vitro DM digestibility (IVDMD) and NDF (IVNDFD) were determined by incubating silage samples in Ankom F57® filter bags for 48 h in a DAYSI-II® incubator, as recommended by the manufacturer. Ruminal inoculum was collected from three slaughtered cows in the Loma Bonita slaughterhouse. Donors grazed tropical pastures.
Total digestible nutrients per hectare (TDN ha-1) was calculated through the equation: %TDN = 87.84 - (0.70 x ADF) (36) and multiplying those values in silages by dry matter yield (DMY) obtained at each cutting height.
Statistical analyses
Data were analyzed for effects of AD, CH and CH x AD using a general linear model (31) as a completely randomized design in a 4 x 3 factorial arrangement with three repeats per treatment: one control (C), three treatments including additive and/or enzyme (F, L and FL) and three CH (12, 25 and 42 cm). Mean comparison was run by least significant difference (LSD), at P≤0.05. Additionally, cell wall fractions and forage yield in response to CH were analyzed by regression analysis.
Results and discussion
Dry matter content
Significant differences in DM content of corn silage were found for the interaction CH x AD (P=0.01). At 12 cm cutting height, C12 (control treatment) showed the highest (P<0.05) and F12 showed the lowest (P<0.05) DM content, with 33.9, 31.2, 30.1 and 28.9% for C12, FL12, L12 and F12, respectively, while for 25 and 42 cm cutting heights, no differences were found (Figure 1).
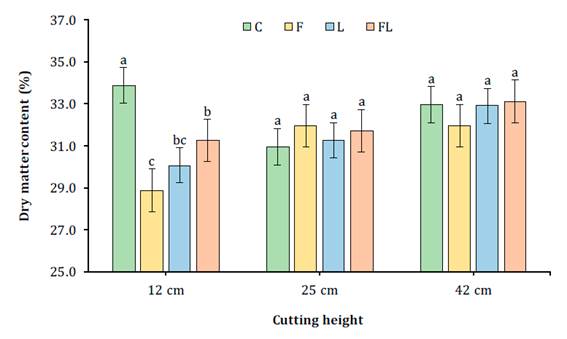
For each cutting height bars with different letter are statistically different (P<0.05). C - control (distilled water only); F - Fibrozyme® (fibrolytic enzymes with xylanase activity); L - Sil-All® (bacterial inoculant for silage based on lactic acid bacteria); FL - mixture of Fibrozyme + Sil-All.
Para cada altura de corte barras con diferente letra son estadísticamente diferentes (P<0,05). C- control (solo agua destilada); F - Fibrozyme® (enzimas fibrolíticas con actividad xilanasa); L - Sil-All® (inoculante bacteriano para ensilaje a base de bacterias ácido-lácticas); FL - mezcla de Fibrozyme + Sil-All.
Figure 1 Dry matter (%) of corn silage in response to additive (C, F, L, FL) and cutting height (12, 25, 42 cm). Figura 1. Materia seca (%) de ensilados de maíz en respuesta al aditivo (C, F, L, FL) y a la altura de corte (12, 25, 42 cm)
Harvesting at higher CH implied an improvement of digestible nutrients per kilogram of DM, then reflected in milk yield, both per area and weight unit. These improvements justify leaving the most fibrous and lignified part of the stem on the field. On the other hand, an increase of 1.7 units (5.2%) in DM content is expected with the increase in CH from 12 to 42 cm, due to higher DM content in cobs compared to leaves or stems (23). In this regard, higher CH results in increased DM content in corn crops for ensiling, explained by a higher stover-to-grain ratio in the ensiled mass (18). Regarding AD, Colombatto et al. (2004) found no effect of added EFE on corn silage DM, with 33.3 and 33.8% average fresh and ensiled corn, respectively.
Dry matter yield (DMY) due to cutting height effect
Average DMY was 10.7 t ha-1. At conventional CH (12 cm) DMY was low (12.5 t ha-1), given three factors: final plant density (52,430 plants ha-1), plant height (198.3 cm) and rainfall, considering water availability is a determining factor in forage yield (30). As expected, DMY was highest (P<0.0001) for the 12 cm as compared to 25 cm and 42 cm CH (Figure 2).
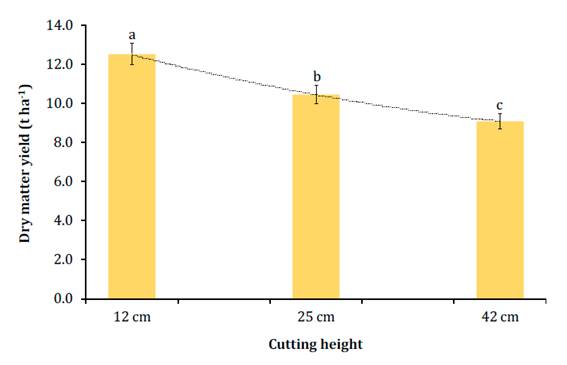
Figure 2 Dry matter yield (t ha-1) of the whole corn plant harvested at three cutting heights (CH). Figura 2. Rendimiento de materia seca (t ha-1) de la planta entera de maíz cosechada a tres alturas de corte
DMY decreases as CH increases, because of the lower proportion of stem in plants harvested at a greater height. On the other hand, DMY is also affected by DM content of the harvested forage, which might vary among different CH (Figure 1, page 132). Both forage DM and forage moisture content, are also related to the amount of stem left on the field at each CH. That is, the higher the CH at harvest (such as 25 cm and 42 cm) the lower the amount of moisture carried within the plant, which in the end is reflected in a higher DM content, since the stem contributes the most to plant moisture (21,40).
DMY is inversely proportional to cutting height. In this study, the greater reduction in DMY occurred between 12 and 25 cm (20.1%) CH; followed by 25 to 42 cm (15.1%); while for CH 12 to 42 cm, DMY was considerably higher (37.3%). The regression analysis showed that for every 1 cm increase in CH above 12 cm, DMY decreases 112 kg ha-1 (R2=0.51; P<0.05). The 37.3% decrease in DMY from 12 to 42 cm is significantly high compared to results published by other research groups. For instance, Wu and Roth (2005) reported a 7.4% reduction in DMY when the CH changed from 17 to 48 cm; Kung et al. (2008) found a DMY drop of 20.1% from 10-15 to 46-51 cm, and Neylon and Kung (2003) reported decreases of 5 to 10% DMY when CH went from 12.7 to 45.7 cm. These reported DMY dissimilarities might be explained by the differences in plant heights among them. For example, Kung et al. (2008) reported an average plant height of 3.04 m, while in the present study, average was 1.98 m. Plant height must be considered before deciding on harvest cutting height.
Chemical composition
pH ranged from 3.86 to 4.43, with an average pH of 4.0, suggesting all silages were well preserved. Silage pH was not affected by the added fibrolytic enzyme, bacterial inoculant or cutting height. The purpose of adding an inoculant or enzyme was to improve forage fermentation by stimulating organic acids production and thus lowering pH (36). However, the addition of L, F or their combination did not stimulate fermentation of WSC due to EFE effect, since final pH remained unaffected, as in previous studies (6,8).
The activity of most EFE improves when pH is above 4.5 (2,38). It has been suggested that EFE work better at a ruminal pH close to neutrality (8). Neylon and Kung (2003), did not find pH changes between corn cutting heights at 12.7 or 45.7 cm, at 34% of DM. Usually, when adding an inoculant, studies focus both on pH and acid concentration (9,24); given the speed at which pH falls and stops enzymatic and bacterial activity plays a key role in avoiding unnecessary nutrient loss. However, in this work pH was not measured at different times. Crude protein (CP) in silages was higher (P<0.01) with L, F or the combination LF, as compared to the control (Table 1), registering an increase of 0.4 to 0.8 %.
Table 1 Crude protein (CP, % of DM) and in vitro digestibility of corn silages harvested at three cutting heights (in cm) and inoculated with lactic acid bacteria and exogenous fibrolytic enzymes. Tabla 1. Proteína cruda (PC, % de la MS) y digestibilidad in vitro de ensilados de maíz cosechados a tres alturas de corte (en cm) e inoculados con bacterias ácido lácticas y enzimas fibrolíticas exógenas
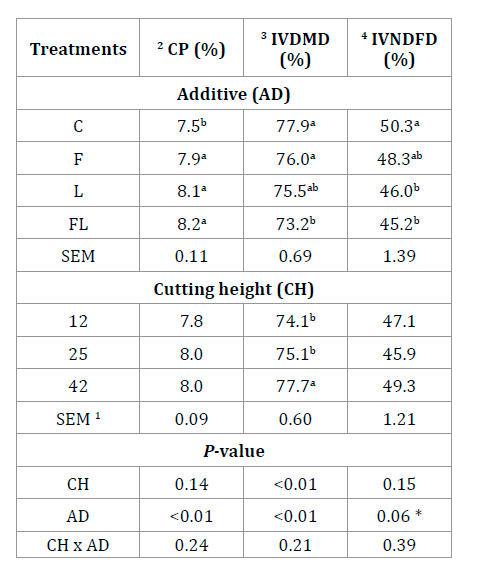
ab For each factor, different letters indicate statistical differences (P≤0.05). 1 SEM - Standard error mean; 2 CP - crude protein; 3 IVDMD - in vitro dry matter digestibility, after 48 h of incubation in the DaisyII equipment; 4 IVNDFD - in vitro neutral detergent fiber digestibility, after 48 h of incubation in the DaisyII equipment; C - control (distilled water); F - Fibrozyme® (fibrolytic enzymes with xylanase activity); L - Sil-All® (bacterial inoculant for silage based on lactic acid bacteria); FL - mixture of Fibrozyme + Sil-All. * - a trend.
ab Para cada factor, diferentes letras indican diferencia estadística (P≤0.05); 1 SEM - Error estándar de la media; 2 CP - proteína cruda; 3 IVDMD - digestibilidad in vitro de la materia seca, después de 48 h de incubación en el equipo DaisyII; 4 IVNDFD - digestibilidad in vitro de la fibra detergente neutro, después de 48 h de incubación en el equipo DaisyII; C - control (solo agua destilada); F - Fibrozyme® (enzimas fibrolíticas con actividad xilanasa); L - Sil-All® (inoculante bacteriano para ensilado a base de bacterias ácido lácticas); FL - mezcla de Fibrozyme + Sil-All. * - una tendencia.
Thus, as previously documented, we assume less proteolysis when these additives are used (14,35). Bacterial inoculants promote faster pH decline in silage with the consequent prevention of more WSC consumption, as well as further proteolysis.
The lower CP content recorded in the control (C) may be related to higher (P<0.05) DM content (32.6, 30.9, 31.4 and 32%) and less DM loss (P<0.05) during fermentation in these silages (0.97, 2.97, 1.85 and 1.87% for C, F, L, and FL, respectively), causing CP dilution. This is also supported by the fact that the treated silage showed lower quality, evidenced by a higher fiber content and a lower digestibility than C silages. Besides, it has been widely stated that protein content in corn silages is not affected by CH at harvest (19,22,38). Finally, average CP content was 7.9%, within the expected range according to NRC (2001).
Cell-wall fractions. The NDF, ADF, hemicellulose, cellulose and ADL, as well as non-fiber carbohydrates (NFC), showed interaction effects (P<0.01). The lower cell wall and higher NFC content occurred in C42 silage (Table 2).
Table 2 Cell wall fractions (% of DM) in corn silages harvested at three cutting heights and inoculated with lactic acid bacteria and exogenous fibrolytic enzymes. Tabla 2. Fracciones de la pared celular (% de la MS) de ensilados de maíz cosechados a tres alturas de corte e inoculados con bacterias ácido lácticas y enzimas fibrolíticas
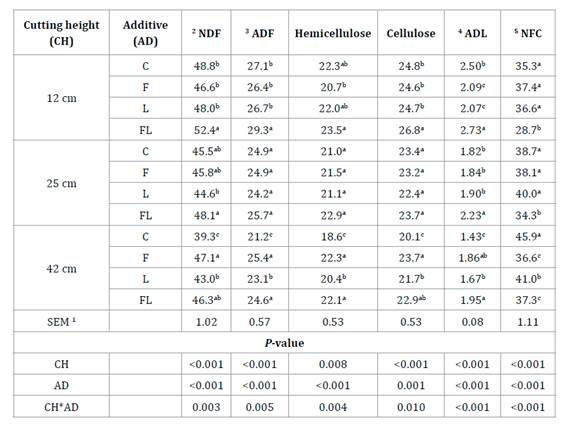
For each cutting height means with different letters are statistically different (P≤0.05). SEM 1- Standard error mean; 2 NDF - neutral detergent fiber; 3 ADF - acid detergent fiber; 4 ADL - acid detergent lignin; 5 NFC - non-fiber carbohydrates [100 - (NDF + CP + ether extract + ash)]; C - control (distilled water); F - Fibrozyme® (fibrolytic enzymes with xylanase activity); L - Sil-All® (bacterial inoculant for silage based on lactic acid bacteria); FL - mixture of Fibrozyme + Sil-All.
Para cada altura de corte, medias con diferente letra son estadísticamente diferentes (P≤0,05). 1 SEM - Error estándar de la media; 2 NDF - fibra detergente neutro; 3 ADF - fibra detergente ácido; 4 ADL- lignina detergente ácido; 5 NFC - carbohidratos no fibrosos [100- (PC + cenizas + grasa cruda + FDN)]; C - control (solo agua destilada); F - Fibrozyme® (enzimas fibrolíticas con actividad xilanasa); L - Sil-All® (inoculante bacteriano para ensilado a base de bacterias ácido lácticas); FL - mezcla de Fibrozyme + Sil-All.
Conversely, the highest cell wall and the lowest NFC contents converged in FL12 silage. In fact, all FL silages showed higher cell walls and lower NFC contents as compared to the remaining treatments (P<0.05). Higher fiber values in FL silages could be consequence of a dilution effect due to a greater loss of WSC occurring in this treatment (15,17). At the CH of 12 cm, hemicellulose and ADL content were lower for the F silages, as compared to C silages, whereas, ADL values of the L silages (P<0.05) were lower than those of the C silages. Cell wall content was affected by cutting height (P<0.05) i.e., the higher cutting height had the lower cell wall content. The latter remained true for the treatments without additives (P=0.05) (Table 2).
In this research, both additives combined resulted in a setback regarding the expected reduction in cell wall fractions. Noteworthy is that EFE activity depends on several factors (type of enzyme, type of forage, pH, temperature, dosage, and others), and if most of these conditions are met, greater enzymatic efficacy may be achieved upon the potentially digestible fraction of the forages.
The NDF, ADF and ADL contents were within the range stated by the NRC (2001) for corn silages with 32 to 38% DM. However, our values were slightly higher than those reported in previous studies (6,22,24,40). Such discrepancies might be explained by environmental differences as is widely known that grasses accumulate more cell walls when grown in warmer climates, such as those from intertropical regions (4), as might be the case for the corn genotype herein considered.
In this study, the use of fibrolytic enzyme negatively impacted silage fiber content and digestibility, probably a consequence of the high xylanase activity presented in the used EFE. It has been observed that enzymes with xylanase activity do not improve DM or NDF biodegradability (11). In contrast, an improvement in NDF degradation occured when EFE had higher endoglucanase activity (11,33). In this regard, Wallace et al. (2001) reported that several products with endoglucanase activity were more effective at stimulating fermentation in corn silage than those with high xylanase activity and low endoglucanase activity.
Finally, Vallejo et al. (2016) concluded that cellulases were more effective than xylanases when added to corn straws. Unlike ferulic acid esterase, xylanase and cellulase cannot hydrolyze the ester bonds between sugars and hydroxycinnamic acids within the cell wall (2). On the other hand, according to the manufacturer the Fibrozyme is recommended for TMR and not for ensiled forage. In this regard, Singh et al. (2018) found that xylanases and cellulases are more effective in TMR than applied on concentrate or forage. Furthermore, LAB are more effective in grasses with lower WSC and low buffering capacity than in forages like corn, sorghum or sugar cane (29). Lastly, LAB do not have a direct influence on forage DM digestibility, but they can promote better silage fermentation.
In vitro DM and NDF digestibility
The IVDMD was affected by both additives and cutting height, while IVNDFD showed a trend (P=0.06) by additive effect (Table 1, page 134). Both IVDMD and IVNDFD, were higher in C and F and lower in FL silages (P<0.05). In this study, IVDMD and IVNDFD followed a similar pattern as compared to cell wall content (Table 2, page 135). The highest digestibility occurred for C silages, where fiber was lower, whereas the opposite occurred for FL silages. Regarding cutting height, IVDMD was higher (P<0.05) for 42 cm (77.7%). IVNDFD was unaltered by cutting height (P=0.15); however, the 42 cm treatment exceeded that of 12 cm by 2.2% (Table 1, page 134).
The highest and lowest IVDMD observed in C and FL respectively, might be related to the lowest (0.97%) and highest (2.97%) DM losses during fermentation. Consequently, less degradable fibrous components increased, either by dilution or by the greater degradation of WSC when additives are used. However, this last hypothesis is weakly supported since pH was not lower. On the other hand, it is also possible that LAB did not use WSC effectively. Sheperd and Kung (1996) observed that after 56 d of fermentation, pH was equal (3.55) for silage treated with EFE compared to untreated, where glucose content represented 0.10 and 0.37% of DM (P<0.05), respectively; suggesting that cell walls were partially hydrolyzed by added enzymes. On the other hand, after 196 d of fermentation with and without EFE, pH was 3.63 and 3.56 (P<0.05) and glucose percentages were 0.09 and 0.10%, respectively. Increases in IVDMD by varying cutting height from 12 to 42 cm, accorded with many studies showing that varying cutting height at harvest implies improvements (12,27) in corn silage digestibility of about 2.5 and 4.7% for IVDMD and IVNDFD, respectively (40).
Corn silage nutritional value could not be improved by EFE, LAB or their combination, since IVDMD and IVNDFD at 48 h of incubation were lower than in the control treatment. Previous studies reported similar effects of EFE on silage (19,24,32); while other studies documented that EFE increased degradation rate after 12 and 24 h of in vitro incubation, but not after 48 or 96 h (6). This is supported by other studies (7), reporting that EFE promote a fast degradation of some fraction of fiber, but have no activity upon the less degradable fraction. Accordingly, digestion rate increases within the first hours of incubation, then decreases until an asymptote (20), given by lack of substrate (6) or inability of enzymes (whether exogenous or ruminal) to degrade that part of the fiber.
Adding EFE and LAB to the forage before ensiling, reduced digestibility of corn silage, particularly when used together. Stokes (1992) also reported this antagonistic effect between EFE and LAB when they are combined. Also, Lynch et al. (2015) reported that adding EFE alone or in combination with ferulic acid inoculant, did not improve corn silage fermentation or nutritional value, and even resulted in negative effects on these parameters.
In this study, neither EFE nor LAB alone, or their combination, showed a positive effect on corn silage quality. These results coincide with other studies on EFE alone (13,19), or combined with LAB (5,24,26). However, more research should confirm these results. Gandra et al. (2017) concluded that the combination of EFE and LAB had a minimal synergistic effect on guinea grass silage quality when added to increase NDF digestibility and decrease silage proteolysis.
In line with previous reports (21), cutting height did not affect IVNDFD (P=0.15) in this study. Nevertheless, most studies do report differences in NDF digestibility. Wu and Roth (2005) observed a 6.7% increase in IVNDFD from 17 to 49 cm cutting heights, while Neylon and Kung Jr (2003) found that digestibility went from 48.7 to 51.5% from 12.7 to 45.7 cm cutting heights. The latter result was attributed to a lower NDF content in silage from corn cut at taller heights. According to Lewis et al. (2004), 66.1, 67.3 or 69.1% of IVNDFD occurred from 15, 30 or 46 cm height, respectively, in three corn hybrids harvested at 35% DM.
Total digestible nutrients (TDN)
An interaction CH x AD (P=0.008) occurred for TDN per hectare (TDN ha-1) (Figure 3).
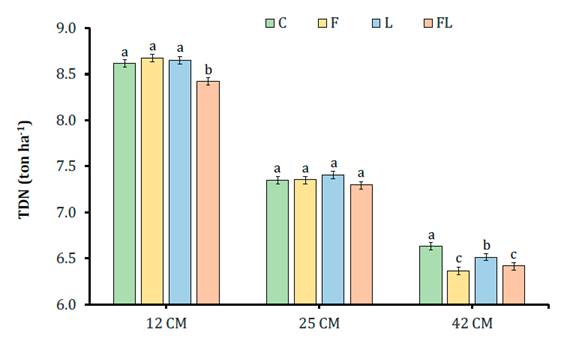
For each cutting height bars with different letters are statistically different (P<0.05). C - control (distilled water only); F - Fibrozyme® (fibrolytic enzymes with xylanase activity); L - Sil-All® (bacterial inoculant for silage based on lactic acid bacteria); FL - mixture of Fibrozyme + Sil-All.
Para cada altura de corte barras con diferente letra son estadísticamente diferentes (P<0,05). C- control (solo agua destilada); F - Fibrozyme® (enzimas fibrolíticas con actividad xilanasa); L - Sil-All® (inoculante bacteriano para ensilaje a base de bacterias ácido-lácticas); FL - mezcla de Fibrozyme + Sil-All.
Figure 3 Total digestible nutrients (TDN) of corn silages treated with different additives (C, F, L, FL) at three cutting heights (CH). Figura 3. Nutrientes digestibles totales (TND) de ensilados de maíz tratados con diferentes aditivos (C, F, L, FL) a tres alturas de corte
The treatment FL12 showed the lowest TDN yield. At 25 cm, no differences were found between treatments, while at 42 cm, TDN was higher in C and L, but lower in F and FL. These data show that F negatively affected TDN ha-1. Moreover, cutting height affected nutrients yield by area reducing 24.5% when cutting height changed from 12 to 42 cm. This decrease is especially important in corn plants with reduced height, as in this study.
Conclusions
Corn silage quality was not affected by adding a bacterial inoculant or a fibrolytic enzyme. The combination of both, inoculant and enzyme, decreased corn silage quality by promoting greater content of cell wall fractions and decreasing dry matter digestibility. Silage quality was greater for the 42 cm cutting height, but this cutting height produced 37.3% less dry matter and 24.5% less nutrients yield. The authors recommend focusing on good practices when ensiling.














