Introduction
Drought represents a massive threat to agricultural productivity (24,35), affecting more than 64% of the world’s land. Almost 70% of Argentina is occupied by drylands, including the extensive Patagonian region that suffers strong desertification processes (48,55). During drought, osmotic stress suppresses overall plant growth (39,43); accelerates the senescence of older leaves (16); reduces the number, size and viability of seeds; and delays germination (9), flowering and fruiting (49,56). Climate change is likely to intensify these factors, further impairing normal growth and reducing plant water use efficiency (12). Crop water use efficiency is therefore a topic worthy of attention (45).
Rhizospheric microorganisms that promote plant growth help plants become estab lished in their environment (32) by enhancing water and nutrient acquisition (34,51), improving homeostasis and tolerance processes (3) and alleviating abiotic stress (21), among other possible mechanisms. One of the most studied fungi types in this regard are mycorrhizal fungi and, more recently, yeasts have also been considered. Mycorrhizal fungi are essential for the development of most plants (52): this symbiosis improves plant estab lishment, enhances plant nutrient uptake (5) and protects host plants from the detrimental effects of osmotic stress caused by water deficit (40). Inoculation with arbuscular mycor rhizae (AM) is a common practice in agriculture and forestry (17). Furthermore, it is known that AM fungi influence and are influenced by the activities of other soil microorganisms (2,5). Microorganisms that facilitate the development and function of mycorrhizal symbiosis are considered mycorrhizal helper microorganisms (1,5). Yeasts have been shown to have growth-promoting properties in plants, including pathogen control (11), phytohormone production (33,47), phosphate solubilization (20), siderophore production (10) and increased AM root colonization (42).
The use of AM fungi and other growth-promoting microorganisms can improve plant establishment and help them cope with stress from factors such as drought and nutrient limitations (6). Native microorganisms have the advantage of being adapted and resistant to local environmental stressors, so could be the most effective when inoculated in plants culti vated in their own environments (37). Inoculation with these microorganisms increases their number in the soil, thus helping maximize their beneficial properties by promoting crop yield (11) and crop tolerance to environmental stress (26).
Microbial communities present complex interactions between species, making it difficult to predict their responses to changes in land use, especially in a context of global change. Major research efforts are underway to generate strategies to combat abiotic stress in plants, and although some are promising, such as farm management practices using breeding and genetic engineering (54), they are time-consuming and expensive. The use of microorganisms for multiple purposes may be an eco-friendly, sustainable and cost-ef fective approach. Studying the interaction between microorganisms and their relationship with plants in an environment with low water availability could help us find low-cost, environmentally friendly biotechnological tools. In Andean Patagonia, several studies have described native rhizospheric yeast communities (27,28) and their physiological character istics that promote in-vitro (29,31) and in-vivo (32) plant growth. Tomato was selected as the object of study here because of its agronomic significance and its role as a model plant in scientific research (14). The objective of the present work was to study how inoculation with arbuscular mycorrhizae and plant growth-promoting yeasts adapted to local condi tions can influence tomato production in water-deficit conditions.
Materials and methods
Experimental design
We designed a tri-factorial pot trial with two irrigation regimes (normal and low), two mycorrhiza levels (with or without addition) and five yeast levels (with one of 4 yeast species or without yeast). The trial comprised 20 treatments with six replicates each (120 plants).
Microbial inocula
The arbuscular mycorrhizal fungus used in our experimental trial was Funneliformis mosseae (F.M.; ex Glommus mosseae). Soil inoculum containing 110 sporocarps per 10 g of soil was produced under greenhouse conditions from trap plants at Estación Experimental del Zaidín, CSIC (Spain).
The yeast strains used belong to the Yeast Collection of the Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, Argentina. They were isolated from rhizo sphere soils of native forests in the Northwest region of Patagonia, Argentina, and identified by Mestre et al. (2011, 2014). Yeast strains were selected based on their plant growth-pro moting traits (29,31) and osmotic tolerance. The four yeasts, Candida aff. ralunensis (C.R.), Candida sake (C.S.), Lachancea nothofagi (L.N.) and Candida oleophila (C.O.), produced auxin-like compounds and tolerated up to 10% NaCl. In addition, C.R. L.S. and L.N. solubi lized phosphates, and L.N. and C.O. produced siderophores. The yeast strains were culti vated at 20 °C for 72 h on solid medium (MYP, g 100mL -1: Malt extract 0.70, Yeast extract 0.05, Soybean peptone 0.23, Agar 1.50). Each yeast strain was suspended in peptone water (1% Soybean peptone) to a turbidity of 0.3 at 600 nm, equivalent to a suspension of 106 cells mL -1. These cell suspensions were used to inoculate the seedlings. The timing and final concentration of the inoculations are detailed in the section “Production Conditions”.
Production conditions
Commercial tomato seeds (Solanum lycopersicum var. platense) were used. The seeds were pre-treated by immersion in 20% sodium hypochlorite for 2 min, followed by triple washing with sterile water, to reduce their microbial load. Three hundred seeds were placed in a culture chamber under controlled conditions (16 hours of light at 25°C, 8 hours of darkness at 22°C) for 4 days, and 140 germinated seeds were then planted in alveolar trays with sterile commercial seedling substrate (with an organic amendment). The Funneli formis mosseae (FM) mycorrhizal inoculum was added to the substrate of half the trays in a 2 % P/V ratio: plants in these trays were thus considered to be inoculated with arbuscular mycorrhiza (F.M.+). The seedlings were incubated in a chamber under controlled conditions (10 hours darkness at 20°C, 14 hours light at 28°C) and periodically watered with sterile water. When the first true leaves appeared (10 to 15 days after germination), 6 ml of yeast inoculum was applied to the stem base of the seedlings as follows: 18 seedlings of each mycorrhizal treatment were inoculated with a single yeast strain (C.R., C.S., L.N. or C.O.), and 18 were inoculated with peptone water without yeast (control, Y-). After 45 days in the germination chamber, all seedlings were transferred to a greenhouse. The greenhouse belongs to the Forestry Department of the Province of Rio Negro and is located in the city of Bariloche, Río Negro, Argentina. Twelve seedlings per treatment were transplanted into pots according to the following criteria: minimum height of 10 cm, at least 3 true leaves, complete root system and well-adhered substrate when detached from the tray insert. Each seedling was placed manually into a 7L pot containing a mixture of perlite, peat and soil in a ratio of 1:1:2. The soil used in the study, sourced from the vicinity of the greenhouse, is commonly employed for cultivating horticultural and forestry seedlings in the area. Typical production conditions were therefore replicated, using soil with its native microbial community. Once in the greenhouse, 60 plants were kept under normal irrigation condi tions (W+) for the entire trial. For the remaining 60 plants (W-) irrigation was discontinued 30 days after transplanting, and a pulse of water (lasting 7 days) was applied only when visible symptoms of plant wilting appeared (loss of stem and leaf turgidity). Normal and low irrigation regimes were set up using a drip irrigation system, and the pots were distributed randomly within each irrigation regime. The low irrigation treatment provided only 15% of the amount of water provided in the normal irrigation treatment.
All the plants were fertilized with commercial Nitrofull (Emerger) three times during the trial, at different phenological stages of the plants: the first was 0.36 g per plant when the plants had no fruit; the second and third doses were 0.8 g per plant, one at the beginning of the fruit production period and the last one close to the end of production. Weed control was carried out every 15 days and axillary buds were eliminated to simulate productive condi tions. The trial was designed such that the production cycle would be completed during the Patagonian growing season, from September (late winter) to April (early autumn), a total of 205 days.
Mycorrhizal colonization: At the end of the production cycle, the root systems of 3 plants from each of the 20 treatments were collected at random. They were first carefully rinsed with tap water and then a portion (2 g per plant) was conserved in alcohol 70% V/V and stained using the modified method of Phillips and Hayman (38). The mycorrhizal status of each plant was determined in fine roots (<2 mm diameter) using an optical micro scope (Olympus BX40). The presence of arbuscules was used as a diagnostic feature of AM presence. For quantification, ten stained root fragments of 1cm length were placed on a slide and observed with 200x magnification, in triplicate, resulting in the observation of at least 300 fields per plant (about 100 fields per preparation). Percentage of AM colonization (AM%) was estimated using the method described by Giovannetti and Mosse (1980).
Growth variables: At the end of the trial all plants were harvested. Plant and root length were recorded with a tape measure (0.1 cm) and stem diameter with a digital caliper (0.01 mm). Aerial and root material was harvested separately, dried at 90 °C to constant weight and then weighed to determine dry biomass to the nearest 0.001 g.
Production variables: Ripe tomatoes were harvested periodically throughout the trial. At the end of the trial all fruits were harvested. The number of flowers and fruits per plant was recorded. The fruit-to-flower ratio (FFR) was calculated as the ratio of the number of fruits to the final number of flowers. Yield was determined by calculating average fresh weight of fruit per treatment.
Statistical analysis
To determine whether the treatments affected the development of the tomato plants, we carried out a three-way ANOVA test, taking into account the following variables: percentage of arbuscular mycorrhiza colonization, root length, stem diameter, dry shoot biomass, dry root biomass, number of flowers, number of fruits, fruit to flower ratio and yield. Data were transformed when necessary to achieve normality: AM% data were transformed with the square root of arcsine, and FFR value and fruit number with the square root. A gamma distri bution was assumed for dry root biomass results. The figures present non-transformed data. Tukey’s post-hoc tests were used to form homogeneous groups when necessary (α = 0.05).
Results
None of the variables analyzed in this trial showed significant interaction between the three factors (3-way interaction), and no interaction was found between yeast inoculation and the addition of AM (co-inoculation) for the variables analyzed.
Mycorrhizal colonization
Neither the yeast treatments nor the interactions had a significant effect on AM%, whereas F.M. addition and irrigation conditions showed significant main effects. The AM% in plants subjected to normal irrigation conditions was significantly higher than in plants under low irrigation conditions (W+ > W-; p = 0.001; Figure 1A); AM% was significantly higher in plants with F.M. than in those without it (F.M.- < F.M+; p = 0.012; Figure 1B).
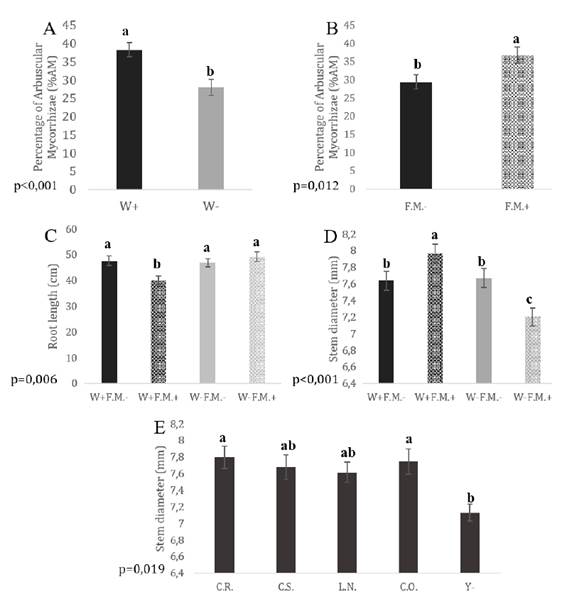
W+: normal irrigation; W-: low irrigation; F.M.-: without Funneliformis mosseae.; F.M.+: with F. mosseae. C.R.: Candida aff. ralunensis; C.S.: C. sake; L.N.: Lachancea nothofagi; C.O.: C. oleophila; Y-: non yeast inoculation. Mean values and standard errors are given for each treatment (bars). Statistically significant differences are indicated by different letters (Tukey’s post-hoc test. p ≤ 0.05).
W+: riego normal; W-: bajo riego; F.M.- : sin Funneliformis mosseae.; F.M.+: con F. mosseae. C.R.: Candida aff. ralunensis; C.S.: C. sake; L.N.: Lachancea nothofagi; C.O.: C. oleophila; Y-: sin inoculación de levadura. Se indican los valores medios y los errores estándar para cada tratamiento (barras). Las diferencias estadísticamente significativas se indican con letras distintas (prueba post hoc de Tukey. p ≤ 0,05)
Figure 1 Effect of irrigation (A) and mycorrhizal inoculation (B) on the percentage of mycorrhizal colonization. Interacting effects of irrigation and mycorrhizal inoculation on root length (C) and stem diameter (D). Effect of yeast inoculation on the stem diameter (E) of tomato plants. Figura 1. Efecto del riego (A) y la inoculación micorrícica (B) sobre el porcentaje de colonización micorrícica. Efectos interactivos del riego y la inoculación micorrícica sobre la longitud radical (C) y el diámetro del tallo (D). Efecto de la inoculación con levaduras sobre el crecimiento del diámetro del tallo (E) de plantas de tomate
Growth variables
Root length was not affected by the individual main factors but was significantly affected by the interaction between irrigation condition and F.M. addition (p = 0.006). Roots were shorter in tomato plants subjected to normal irrigation conditions with F.M. than in any other treatment (W+F.M.+ < W+F.M.-, W-F.M.+, W-F.M.-; Figure 1C).
Stem diameter was affected by yeast inoculation as a main effect (p = 0.019): tomato plants inoculated with C.R. or C.O. showed larger stem diameters than plants without yeast inoculation (C.R. = C.O. > Y-; Figure 1E, page 145). Stem diameter was also affected significantly by the interaction between irrigation condition and F.M. addition (p ≤ 0.001): the highest value was obtained with F.M. and normal irrigation, intermediate values were observed for both irrigation treatments without F.M., and the lowest values were obtained with F.M. and low irrigation (Figure 1D, page 145).
In the case of dry shoot biomass, F.M. addition was the only factor that generated signif icant differences (p = 0.015): plants without F.M. showed higher values than those with FM (F.M- > F.M.+). The only factor that generated significant differences for dry root biomass was the irrigation condition (p ≤ 0.001): plants under low irrigation conditions had higher root dry biomass than those receiving normal irrigation (W- > W+).
Production variables
Flower numbers showed a significant interaction between irrigation and yeast inocu lation (p = 0.012). The number of flowers was higher in plants exposed to normal irrigation conditions and inoculated with C.S. than in plants under low irrigation conditions inocu lated with the same yeast strain (W+C.S. > W-C.S.; Figure 2A, page 147). On the other hand, of the normally irrigated plants those inoculated with C.R. and C.S. showed higher numbers of flowers than those inoculated with C.O. Of the plants with reduced irrigation, those inoc ulated with C.R. had higher numbers of flowers than those inoculated with C.S. The main effect of F.M. addition showed significant differences in the number of fruits (p < 0.001): plants with F.M. produced 55% more fruit than those without it (F.M.- < F.M.+; Figure 2B, page 147). The number of fruits also showed a significant interaction between the irrigation and yeast factors (p = 0.025). Plants inoculated with C.R. had a higher number of fruits under normal irrigation than under low irrigation (W+C.R. > W-C.R.; Figure 2C, page 147), and plants inoculated with C.S. had a higher number of fruits than plants inoculated with C.R. under low irrigation conditions (W-C.S. > W-C.R.; Figure 2C, page 147).
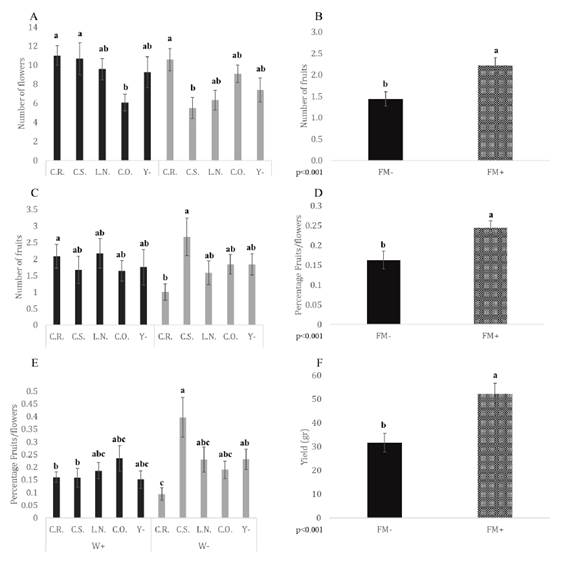
W+: normal irrigation; W-: low irrigation; F.M.-: without Funneliformis mosseae.; F.M.+: with F. mosseae. C.R.: Candida aff. ralunensis; C.S.: C. sake; L.N.: Lachancea nothofagi; C.O.: C. oleophila; Y-: without yeast inoculation. Mean values and standard errors are given for each treatment (bars). Statistically significant differences are indicated by different letters (Tukey’s post-hoc test. p ≤ 0.05).
W+: riego normal; W-: bajo riego; F.M.- : sin Funneliformis mosseae.; F.M.+: con F. mosseae. C.R.: Candida aff. ralunensis; C.S.: C. sake; L.N.: Lachancea nothofagi; C.O.: C. oleophila; Y-: sin inoculación con levaduras. Se indican los valores medios y los errores estándar para cada tratamiento (barras). Las diferencias estadísticamente significativas se indican con letras distintas (prueba post hoc de Tukey. p ≤ 0,05)
Figure 2 Interactive effects of irrigation and yeast inoculation on A) Number of flowers C) Number of fruits and E) Fruits/flowers ratio. Effect of mycorrhizal inoculation on B) Number of fruits D) Ratio fruits/flowers F) Yield. Figura 2. Efectos interactivos del riego y la inoculación de levadura sobre A) Número de flores C) Número de frutos y E) Proporción frutos/flores. Efecto de la inoculación micorrícica sobre B) Número de frutos D) Proporción frutos/flores F) Rendimiento
Funneliformis mosseae addition showed significant differences as a main effect for the fruit-to-flower ratio (p < 0.001), which was higher in plants with F.M. than in those without it (F.M.- < F.M.+; Figure 2D, page 147).
The FFR also showed significant interaction between irrigation and yeast inoculation (p = 0.005). For plants inoculated with C.R., the FFR was significantly higher under normal than low irrigation conditions (W+C.R. > W-C.R.; Figure 2E, page 147). In contrast, for plants inoculated with C.S. the FFR was lower in plants under normal irrigation conditions than low (W-C.S. > W+C.S.). Plants under low irrigation conditions and inoculated with C.S. rendered the highest FFR.
Yield showed significant differences for F.M. addition as a main effect (p < 0.001): plants with F.M. showed a 65% higher yield than plants without F.M. (F.M.- < F.M.+; Figure 2F, page 147).
Discussion
Our results indicate no three-way interaction between factors for any of the variables measured. Statistical significance was observed for single main effects of the factors or pairwise interactions, one of the interacting factors being the irrigation condition. The irri gation regime therefore seems to be the main source of variation for most of the variables analysed. Plants subjected to a low irrigation regime received 85% less water and showed symptoms of osmotic stress, such as a decrease in stem diameter and number of flowers. This may be related to growth inhibition due to osmotic stress. Hydric stress decreases stem and leaf growth and accelerates senescence and abscission in older leaves (16). Under water deficit conditions, plants generate strategies to modulate their soil water uptake capacity, such as lateral root development or main root elongation (9,22,44); this explains the increase in root length and root dry biomass observed under low irrigation conditions.
The effect of drought on mycorrhizal symbiosis was poorly established since we found negative, positive and even neutral effects (4,15,25,57). Drought effects on the estab lishment and colonization of mycorrhizal fungi depend on several conditions, such as plant and fungal species, environmental conditions and stress levels (4). In our work, limiting irrigation negatively affected mycorrhizal colonization.
Other studies have reported that co-inoculation of arbuscular mycorrhizal fungi and yeasts can have positive effects on plant growth and development (57). These effects may be related to beneficial interactions between the AM fungi and yeasts, such as nutrient solubili zation and improved plant resistance to stress. In our study, however, co-inoculation had no significant effect on tomato plants. This could be due to the particular yeast and AM species used, soil conditions or production conditions.
Mycorrhizal colonization was observed in all the plants studied, demonstrating that mycorrhizal communities in Patagonian soils are capable of colonizing agriculturally important plants such as the tomato. Addition of F. mosseae increased mycorrhizal colo nization, which may be attributed to higher inoculum pressure, the highest infectivity of F. mosseae, or a beneficial synergistic effect of both AM communities on these annual plants. The addition of F. mosseae increased productive parameters such as fruit number, FFR and yield in tomato plants. Arbuscular mycorrhiza hyphae can penetrate soil pores and extend beyond the root zone, increasing the soil volume to be explored and the possibility of better nutrient uptake (41,46). Additionally, AM fungi are known to influence the nutrient balance of plants, including carbohydrate balance (7) and hormone production (50), two factors that affect flowering and fruit set (8). This indicates that increased AM colonization, in this case, the addition of F. mosseae, can positively influence productive parameters, both under normal irrigation conditions and in situations of water shortage.
The relationship between Patagonian yeasts and native mycorrhizal colonization has been reported in studies such as Mestre et al. (2017), where a tendency of increased colo nization of native mycorrhizae was observed in poplars inoculated with the native yeast Tausonia pullulans. Fracchia et al. (2003) recorded an increase in mycorrhizal colonization in soybean (Glycine max) and red clover roots after double inoculation of F. mosseae and Rhodotorula mucilaginosa, when the yeast was inoculated before F. mosseae. Our results show that yeast inoculation did not significantly affect the percentage of AM colonization; however, there was a tendency of increased mycorrhizal colonization in plants inoculated with C. sake, without F. mosseae, under both irrigation conditions. This suggests a possible mycorrhizal helper effect of C. sake on native mycorrhizal fungi present in Patagonian soils. Further study should be carried out on this relationship, considering factors such as the concentration of each microorganism, the location, timing and frequency of inoculations, the order of inoculation of the microorganisms, and a combination of these factors, to enhance understanding and enable improvements to be made in agricultural production strategies.
Enhancing the ability of native mycorrhizal fungi to colonize economically important crops could be an alternative to using external mycorrhizal inoculum, which has to be purchased by producers and adds to production costs. From an environmental point of view, using native yeasts to improve native mycorrhizal colonization may be advantageous in that the introduction of foreign microorganisms can be avoided. Inoculation with C. aff ralun ensis and C. oleophila led to significantly larger stem diameters than in plants without yeast, under both irrigation conditions and F. mosseae addition. Plants with larger stem diameters are less susceptible to environmental stress after transplanting (53). Stem diameter is a general measure of plant resistance to drought (19), and is often correlated with transplant vigor (23). The greater the vigor of the plants, the more resilient they will be in adverse conditions and the more capable of producing a large quantity of fruit. Therefore, inocu lation of either of these two yeasts can enhance overall plant resistance by increasing plant vigor. Inoculation with C. aff ralunensis and C. oleophila are proposed as a complement to inoculation with F. mosseae, as a way of improving plant performance under water deficit conditions, in which the results of F. mosseae addition were not as good as under normal irrigation. We believe that one possible mechanism by which C. aff. ralunensis and C. sake promoted plant productivity is linked to their ability to solubilize inorganic phosphate. Argentine Patagonia has Andisol soils characterized by high phosphorus retention (36); the presence of solubilizing microorganisms is, therefore, crucial as they make this nutrient available for plant uptake, improving plant nutrition. The direct characteristics of C. sake as a plant growth promoter have been reported in the work of Gollner et al. (2006), where inoculation with C. sake increases the biomass of maize (Zea mays) plants. Although in our research C. sake does not present statistically significant differences compared to control plants, we observed that under water deficit conditions it reached the highest values in productive parameters such as fruit number and FFR. This suggests a promising trend, although not statistically significant, indicating possible potential as a growth promoter under water stress. Continuing research to explore this trend is required to confirm its viability as a beneficial solution under water scarcity conditions.
Conclusions
Our findings reveal that adding F. mosseae significantly increases arbuscular mycorrhizal colonization and improves several productive parameters in tomato plants, both under normal and limited irrigation conditions. The use of indigenous rhizospheric yeasts such as C. aff. ralunensis and C. oleophila, is proposed for the cultivation of more robust plants, not only in conventional irrigation systems but also in situations of water scarcity. These findings indicate that employing indigenous microorganisms could be a promising alter native to external inoculants, potentially reducing production costs and obviating the need to introduce foreign microorganisms into the environment. Arbuscular mycorrhizae and yeast inoculation could be effective in improving crop yields and increasing plant resistance to water stress. Nevertheless, additional research is necessary to further understand these processes and optimize their practical application in agriculture.














