Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cirugía
versión impresa ISSN 2250-639Xversión On-line ISSN 2250-639X
Rev. argent. cir. vol.116 no.2 Cap. Fed. jun. 2024 Epub 01-Jun-2024
http://dx.doi.org/10.25132/raac.v116.n2.1780
Original article
New perspectives in the management of low-risk thyroid cancer. Experience with thyroid lobectomy in a cohort of 114 patients
1Servicio de Cirugía de cabeza y cuello, Hospital Universitario Austral. Buenos Aires. Argentina
2Servicio de Endocrinología, Hospital Universitario Austral. Buenos Aires. Argentina
Background:
The treatment of low-risk differentiated thyroid cancer (DTC) is still a matter of debate. Over the past few decades, there has been a shift towards a more personalized approach, tailored to the individual risks of each patient and tumor. The current practice guidelines recommend lobectomy in selected patients, with low risk of recurrence.
Objective:
To describe the results of thyroid lobectomy in a cohort of patients with DTC with low risk of recurrence.
Material and methods:
We conducted a descriptive and observational study. The medical records of patients with DTC who underwent thyroid lobectomy between January 2015 and April 2023 were retrospectively reviewed.
Results:
A total of 114 patients were operated on; mean age was 44 ± 12 years and 90 (79%) were women. The median tumor size was 9.4 mm (IQR 25-75% 7-13 mm), and 103 nodules (90%) were solid on ultrasound. Only 2 patients with vascular invasion involving > 4 vessels required completion thyroidectomy. There were no major complications and only one patient developed temporary recurrent laryngeal palsy. There were no locoregional or distant recurrences during mean follow-up of 33.4 months.
Conclusion:
Thyroid lobectomy for low-risk DTC had low morbidity and no recurrences in the series presented. The rigorous selection of patients and the interaction of a multidisciplinary team are considered essential for the successful implementation of this therapeutic approach.
Keywords: thyroid carcinoma; low risk; thyroid lobectomy; recurrence risk; ultrasound imaging; patient selection
Introduction
The management of low-risk differentiated thyroid cancer (DTC) remains controversial because of the indolent nature of the disease and the challenge of balancing morbidity of treatments with the risk of disease progression. In recent decades, a better understanding of the biology of thyroid cancer and its classification according to risk of recurrence have led to more conservative and personalized treatment options1,2.
After the publication of Mazzaferri et al.3, total thyroidectomy with systematic radioactive iodine administration became the gold standard for almost 40 years. This strategy was supported by the work of Bilimoria et al. in 2007, which was in turn questioned due to the lack of relevant data4. However, this paradigm lost support with the analysis of 62,000 patients by Adam et al.5 who found no difference in survival between lobectomy and total thyroidectomy after adjustment for comorbidities and tumor-specific variables. This finding has been confirmed by numerous subsequent retrospective studies6, 7,8, 9. The strategy for administering radioactive iodine was modified after some retrospective studies failed to show a benefit in recurrence or mortality in the low-risk group10. A recent French prospective study (ESTIMBL 2) of 776 patients reported no inferiority in survival in stage I and II with and without the administration of 30 mCi of radioactive iodine after total thyroidectomy11.
Given that most low-risk DTCs have a favorable prognosis, minimalistic strategies have become more prevalent in recent years. These approaches offer lower morbidity rates while maintaining similar oncologic outcomes and improving quality of life1.
In this context, thyroid lobectomy has consolidated its role for the treatment of low-risk DTCs in appropriately selected patients, alongside other nonsurgical options such as active surveillance12 and percutaneous thermal ablation13.
The aim of the present study was to report the outcomes of thyroid lobectomy as the primary surgical intervention for low-risk TDC.
Material and methods
We conducted a retrospective cross-sectional study of a cohort of 114 patients with low-risk DCT treated with lobectomy plus isthmusectomy between January 2015 and April 2023. Data was obtained from the electronic medical records. The study protocol was approved by the Unit of Clinical Research.
We included adult patients with no significant comorbidities and with the following eligibility criteria: thyroid nodules with fine needle aspiration cytology class V and VI of the Bethesda system, with a size < 4 cm on ultrasound, unifocal, without clinical or ultrasound evidence of contralateral nodules and/or lymph node disease, without macroscopic extrathyroidal extension or distant disease. Patients with benign or indeterminate preoperative cytology, with intraoperative or postoperative findings meeting the above criteria were also included. The exclusion criteria were age < 18 years and a history of exposure to radiation.
We recorded demographic data, ultrasound characteristics, cytology, type of approach and complications, pathological findings, histologic risk factors, risk of recurrence, associated thyroiditis, TNM stage, long-term outcome, and need for postoperative hormone therapy.
The surgery performed was a thyroid lobectomy plus isthmusectomy using either a conventional cervical incision or transoral approach. Prophylactic central neck lymph node dissection was not performed in accordance with our practice standards and current clinical practice guidelines. The central compartment was systematically explored during lobectomy. All lymph nodes found during exploration that were not detected by preoperative ultrasound underwent frozen section biopsy. If lymph nodes were positive, completion thyroidectomy with central lymph node dissection was performed, and the patient was excluded from the present study.
The pathological examination of the surgical specimen reported the morphologic characteristics, presence of minimal capsular invasion, multifocality, vascular invasion (< than 4 vessels or ≥ 4 vessels), lymph node metastases not diagnosed on intraoperative examination, and the percentage of cells with high-risk characteristics in the case of aggressive variants (tall cell variant).
The risk of recurrence was classified according to the American Thyroid Association (ATA) categories. The low-risk category included papillary thyroid cancer < 4 cm, classic variant, no vascular invasion or < 4 vessels, clinical N0 or ≤ 5 pathologic N1 micrometastasis all < 2 mm, and follicular thyroid cancer with capsular invasion or minimal vascular invasion (< 4 vessels).
Intermediate risk corresponded to papillary thyroid cancer with vascular invasion, follicular thyroid cancer with vascular invasion > 4 vessels, microscopic extrathyroidal extension, N1 (> 5 lymph nodes, all < 3 cm), and aggressive histology with tall cells > 30%. Staging and mortality risk were assessed using the TNM staging system (8th edition). Both classifications are based on preoperative and intraoperative parameters to tailor the extent of surgery to each patient and tumor characteristics.
All the statistical calculations were performed using STATA BE 17.0, software package with perpetual software license for the Department of Academic Development, Hospital Universitario Austral.
Continuous variables were expressed as mean and standard deviation (SD), or median and interquartile range (IQR) 25-75%, according to their distribution. Categorical variables are presented as frequencies and percentage.
Results
Our experience with thyroid lobectomy for the treatment of DTC started in 2015 and gradually increased to reach 29.3% of cases in 2022 (Figure 1). During this period, 924 thyroidectomies were performed, 583 of which were for DTC. Out of these, 469 were total thyroidectomies, 110 were lobectomies with isthmusectomy, and 4 were isthmusectomies as the only resection. In total, lobectomies and isthmusectomies accounted for 19.5% of thyroid surgeries for DTC during the period analyzed. The mean age was 44 ± 12 years, 90 patients (79%) were women, the median tumor size was 9.4 mm (IQR 25-75% 7-13 mm), and 103 nodules (90%) were solid on ultrasound (Table 1).
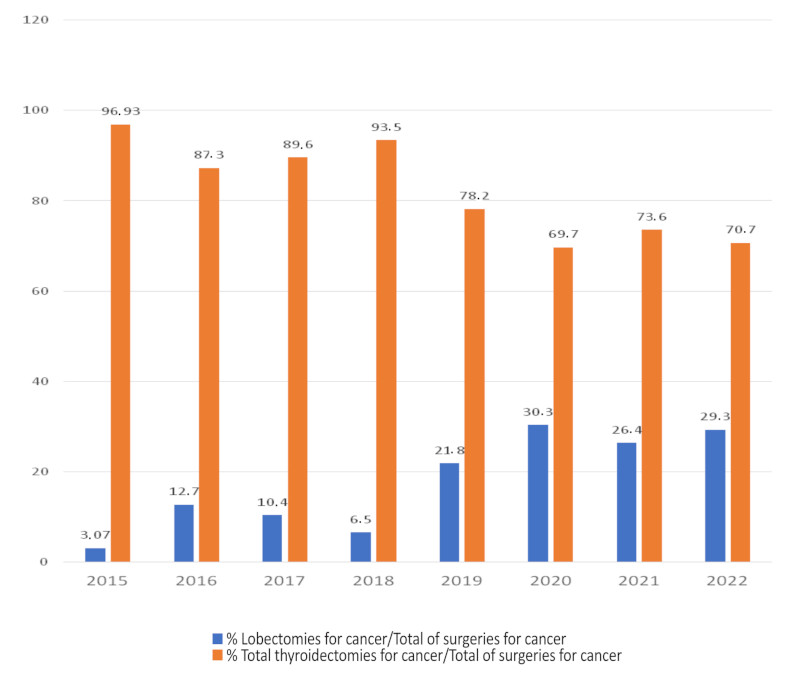
FIGURE 1 Thyroid lobectomies for DTC relative to total thyroidectomies for cancer between 2015 and 2022
The preoperative cytology report according to the Bethesda system was category V and VI in 87 patients (76%). Twenty-seven patients with benign or indeterminate preoperative cytology were included in this series because the diagnosis of cancer was made after frozen section biopsy or definitive histology and total thyroidectomy was not performed (Table 1).
TABLE 1 Ultrasound and cytologic characteristics of the tumors
| n = 14 | % | |
|---|---|---|
| Ultrasound | ||
| Solid | 103 | 90.35 |
| Cystic | 2 | 1.75 |
| Mixed type | 9 | 7.89 |
| Bethesda category | ||
| I | 2 | 1.75 |
| II | 8 | 7.02 |
| III | 6 | 5.26 |
| IV | 11 | 9.65 |
| V | 20 | 17.54 |
| VI | 67 | 58.77 |
Sixty-five percent of the patients (74/114) corresponded to microcarcinomas; only 2 patients (1.7%) were T3 (due to size > 4 cm). Micrometastases in the central compartment were found in 13 patients (11.4%), 95% (108/114) were stage I and only 2 patients corresponded to stage II because they were > 55 years with N1a (Table 2).
TABLE 2 TNM staging system and risk for recurrence according to the ATA classification
| TNM staging system | n | % |
|---|---|---|
| T1a | 74 | 64.91 |
| T1b | 31 | 27.19 |
| T2 | 7. | 6.14 |
| T3 | 2 | 1 |
| N0 | 60 | 52.63 |
| N1a | 13 | 11.40 |
| Nx | 41 | 35.96 |
| Stages | ||
| I | 112 | 98.25 |
| II | 2 | 1.75 |
| Risk of recurrence according to ATA | ||
| Low | 109 | 95.61 |
| Intermediate | 5 | 4.39 |
Lobectomies and isthmusectomies were performed by conventional cervical incision in 93 cases (82%) and by transoral endoscopic thyroidectomy vestibular approach (TOETVA) in 21 patients (18%). In 48 patients (42%), some type of central lymphadenectomy was added, with a median of 2 resected nodes (IQR 25-75% 1-3 nodes). Delayed completion thyroidectomy was performed in two patients (1.8%) with a diagnosis of follicular thyroid cancer with vascular invasion involving > 4 vessels. There were no tumor findings in the resected residual lobes in either case. One of the patients underwent TOETVA in both procedures.
The definitive pathological results are shown in Table 3.
TABLE 3 Pathological findings in 114 patients
| n | % | |
|---|---|---|
| Histological types | ||
| Papillary | 85 | 74.56 |
| Follicular | 5 | 4.39 |
| Follicular variant of papillary thyroid carcinoma | 14 | 12.28 |
| Hürtle cells carcinoma | 1 | 0.88 |
| High-risk variants | 8 | 7.02 |
| NIFPT | 1 | 0.88 |
| Multifocality | 16 | 14.04 |
| Vascular invasion | ||
| Minor (less than 4 vessels) | 6 | 5.26 |
| Extensive (4 vessels o more) | 2 | 1.75 |
| Hashimoto’s thyroiditis | 47 | 41.22 |
NIFPT : Non Invasive Follicular Thyroid Neoplasm with Papillary-like nuclear features.
The risk of recurrence was low in 95.6% of patients, and 5 patients were considered in the intermediate risk category based on the findings in the delayed pathology report. In these patients total thyroidectomy was not performed based on the recommendation of the treating team or patient preference. Complications included temporary recurrent laryngeal palsy in one case and mild ecchymosis in 15 of the 19 patients who underwent the transoral approach. There were no recurrences in the contralateral lobe or in the central and lateral neck region, nor distant metastases, and all patients remained disease-free until the end of this study. Sixty percent of the patients who underwent surgery required thyroid hormone replacement, with dosages ranging from 50 to 150 µg/24 hours. Mean follow-up was 33.4 months (range: 2 to 101 months).
Discussion
The management of low-risk DTC is constantly evolving and raises uncertainty as higher level of evidence supports the use of less invasive therapeutic strategies to minimize morbidity, avoid overtreatment, and prevent unnecessary interventions14.
The present study provides additional evidence for the efficacy of thyroid lobectomy in the treatment of low-risk DTC in appropriately selected patients. The results for recurrence-free survival were excellent and consistent with those reported by other high-volume centers9,15,16. The absence of major complications and disease recurrence is noteworthy. However, we are aware that a percentage of patients had inadequate follow-up.
During the study period, there was a significant increase in the number of lobectomies performed for DTC. This trend was also observed in the analysis of a group of general hospitals in the United States, which reported that 22% of thyroidectomies performed in these centers were lobectomies after the publication of the ATA (American Thyroid Association) guidelines17. In a high-volume center, Untch et al. report 15% of lobectomies in their series of thyroidectomies for cancer15, a figure similar to the average number of lobectomies performed by our group (19%) during the period analyzed. This percentage is relatively low considering that the guidelines recommend lobectomy in tumors < 4 cm18. However, some groups advise against lobectomy in tumors > 2 cm because survival is higher after total thyroidectomy but not for tumors
< 2 cm, in which there are no differences in terms of survival between both procedures19. In our experience, we have been very restrictive in selecting candidates for lobectomy based on size. More than 90% of cases were T1 tumors (< 2 cm) and only 6% were T2 tumors (> 2 cm), with 96% corresponding to a low risk of recurrence.
The finding of high-risk histologic features that were not diagnosed preoperatively, such as aggressive histology, extrathyroidal extension, lymph node metastasis, vascular invasion, and multifocality, are reasons to question lobectomy20.
Lymph node micrometastases are a relatively common finding (11% in our experience), but are of little clinical significance, with a risk of recurrence similar to that of patients without micrometastases, since not all micrometastases progress to structural disease and therefore do not indicate the need for total thyroidectomy21. For this reason, thyroidectomy was not performed in any of the patients with micrometastases. Minor extrathyroidal extension is also a low-risk criterion. In a meta-analysis of 23 studies22, the risk of recurrence was very low (3.5%), and the AJCC (American Joint Committee on Cancer), 8th edition, does not consider it a risk factor for upstaging23. Therefore, its presence does not change the indication for lobectomy. Multifocality, which occurs in 13 to 71% of cases24,25 in association with papillary thyroid cancer, is another finding that casts doubt on the advisability of lobectomy. In a meta-analysis of 21 articles, Joseph et al. reported that multifocality is a significant risk factor for disease progression and increases the risk of recurrence, and that total thyroidectomy is recommended in these cases26. However, in a recent study by Harries et al., they did not find differences in the likelihood of contralateral disease or regional recurrence and considered lobectomy as a valid procedure in T1-T2 N0M0 papillary thyroid cancer patients, regardless of the presence of multifocal disease27. In line with this approach, the finding of multifocality did not change the indication for lobectomy in our study.
Since the publication of the ATA guidelines in 2015, several studies have reported that 30-59% of patients would require completion thyroidectomy if initially treated with lobectomy due to the detection of high-risk histological features in the pathological examination of the surgical specimen28. In our cases, this number was very low (1.8%), which is consistent with the experience of Untch et al., who performed completion thyroidectomy after initial lobectomy in only 3% of the cases over a 10-year period. Of these cases, only 34% had contralateral tumors, and 90% were microcarcinomas with no prognostic significance. There are only two categorical indications for completion thyroidectomy: vascular invasion > 4 vessels and aggressive histology (tall cells) if the percentage of cells with these characteristics is > 30%. In 2017, the 4th edition of the World Health Organization classification of tumors of endocrine organs considered a tall cell component > 30% as a criterion for tall cell variant29. These findings occur in no more than 3-4% of cases and are almost never isolated, since they are usually associated with extrathyroidal extension or positive lymph nodes or both, features that can be identified preoperatively and their presence rules out the indication for lobectomy.
It is worth noting that the group of Ganly et al. at the Memorial Sloan-Kettering Cancer Center recommends treating patients with less than 30% tall cells as classic papillary thyroid cancer. They also suggest avoiding aggressive treatment in patients with these features if they are not accompanied by other high-risk features30.
The decision to perform completion thyroidectomy after lobectomy is also closely related to the decision of the treatment team regarding the need for postoperative radioactive iodine administration. In our center, the indication for radioactive iodine ablation is very selective and limited to some intermediate-risk and high-risk patients.
Achieving good results with a minimalist approach is directly related to selecting the right patients before surgery. Ideal candidates are those with microcarcinomas in a favorable location (center of the lobe), a single nodule, no evidence of thyroiditis on ultrasound, and those located in the thyroid isthmus, preferably with a TSH level < 2 µIU/L. Appropriate but not ideal candidates for lobectomy include intrathyroid carcinomas measuring 1-4 cm, with the presence of antibodies or signs of thyroiditis on ultrasound, lymph node micrometastases < 2 mm and > 5 in number, minor vascular invasion < 4 vessels, and aggressive histology if the percentage of cells with high-risk features is < 30%12. Ultrasound can preoperatively diagnose most of the features inappropriate for lobectomy, except for vascular invasion and aggressive histology. Our low incidence of reoperation can be explained by the intensive use of this tool, which, together with an experienced operator, allowed a rigorous selection of candidates for lobectomy. Systematic intraoperative exploration of the central compartment and frozen section biopsy play an additional role in the presence of suspicious lymph nodes. According to Raffaelli et al.31, intraoperative biopsy has a sensitivity of 81%, a specificity of 100%, and an accuracy of 90%. This allows for modulation of the extent of surgery and avoidance of inadequate lobectomies, as was done in this series. Patients considered for lobectomy should be informed and accept a risk of recurrence of up to 6%, with the possibility that the preoperative plan may need to be modified or reoperation may be required if postoperative histologic findings warrant it16.
More recently, interest in cosmetic outcomes has led to the development of minimally invasive techniques such as the transoral approach first reported by Anuwong in 201632 to reduce or avoid visible scarring.
Based on our experience with the transoral approach, 19% of the lobectomies in this series were performed using this technique. Although the literature on this topic is limited and the number of patients studied is small, there is a consensus that the transoral approach is a safe method with results similar to those of the conventional approach in highly selected patients. This is particularly true for microcarcinomas that meet the same requirements as those for lobectomy through cervical incision33, 34, 35. Our experience with lobectomies using TOETVA for DTC is consistent with this recommendation, as all procedures were successfully performed with excellent cosmetic results, without recurrence or major complications, except for mild submental ecchymosis in some patients36. However, more prospective comparative studies are needed to ensure equivalent results to the conventional approach.
This study has limitations due to its small sample size, retrospective nature, and incomplete follow-up in some patients. However, it also has strengths, such as a rigorous selection process for lobectomy candidates, performed by the same team in a multidisciplinary context with endocrinologists and surgeons working together to manage low-risk differentiated carcinoma. Achieving good results with precision, personalized, and patient-centered medicine requires a comprehensive discussion of the disease characteristics, therapeutic options, expected quality of life, and patient preferences.
In conclusion, the results of the present study are consistent with those published in the international literature on thyroid lobectomy as a valid alternative to total thyroidectomy for the surgical treatment of DTC with low risk of recurrence in strictly selected patients. This option offers optimal results in terms of recurrence-free survival and absence of complications. It also suggests that the transoral approach is a safe technique with results similar to those of the conventional approach, although more experience and follow-up are needed to ensure the oncologic efficacy of this technique.
Referencias bibliográficas /References
1. Tuttle M, Alzahrani A. Risk stratification in differentiated thyroid cancer: from detection to final follow-up. J Clin Endocrinol Metab 2019; 104: 4087-4100. [ Links ]
2. Zheng W, Li J, Lv P, Chen Z, Fan P. Treatment efficacy between total thyroidectomy and lobectomy for patients with papillary thyroid microcarcinoma: A systemic review and meta-analysis. Eur J Surg Oncol. 2018; 44:1679-84. [ Links ]
3. Mazzaferri E, Jhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994; 97:418-28. [ Links ]
4. Bilimoria K, Bentrem D, Ko C, Stewart A, Winchester D, Talamonti M, et al. Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246:375-81. [ Links ]
5. Adam M, Pura J, Gu L, Dinan M, Tyler D, Reed S, et al. Extent of surgery for papillary thyroid cancer is not associated with survival: an analysis of 61,775 patients. Ann Surg. 2014;260:601-5. [ Links ]
6. Matsuzu K, Sugino K, Masudo K, Nagahama M, Kitagawa W, Shibuya H, et al. Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38: 68-79. [ Links ]
7. Nixon I, MD, Ian Ganly I, Patel S, Palmer F, Whitcher M, et al. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151:571-9. [ Links ]
8. Raffaelli M, Tempera S, Sessa L, Lombardi C, De Crea C, Bellantone R. Total thyroidectomy versus thyroid lobectomy in the treatment of papillary carcinoma. Gland Surg. 2020;9 (Suppl 1): S18-S27. [ Links ]
9. Song E, Han M, Oh H, Kim W, Jeon M, Lee Y, et al. Lobectomy is feasible for 1-4 cm papillary thyroid carcinomas: a 10-year propensity score matched-pair analysis on recurrence. Thyroid. 2019; 29: 64-70. [ Links ]
10. Nixon I, Ganly I, Patel S, Palmer F, Di Lorenzo M, Grewal R, et al. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on Memorial Sloan-Kettering Cancer Center risk group stratification. Thyroid. 2013;23:683-94. [ Links ]
11. Leboulleux S, Bournaud C, Chougnet C, Zerdoud S, Al Ghuzlan A, Catargi B, et al. Thyroidectomy without radiiodine in patients with low-risk thyroid cancer. N Engl J Med. 2022;386:923-32. [ Links ]
12. Tuttle M, Zhang L, Shaha A. A clinical framework to facilitate selection of patients with differentiated thyroid cancer for active surveillance or less aggressive initial surgical management. Expert Rev Endocrinol Metab. 2018;13:77-85. [ Links ]
13. Tuttle M, Li D, Ridouani F. Percutaneous ablation of thyroid cancer. Endocr Relat Cancer. 2023;30:e220244. [ Links ]
14. Ward L, Scheffel R, Hoff A, Ferraz C, Vaisman F. Treatment strategies for low-risk papillary thyroid carcinoma: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM). Arch Endocrinol Metab. 2022;66:522-32. [ Links ]
15. Untch B, Palmer F, Ganly I, Patel S, Tuttle M, Shah J, et al. Oncologic outcomes after completion thyroidectomy for patients with well-differentiated thyroid carcinoma. Ann Surg Oncol. 2014;21:1374-8. [ Links ]
16. Vaissman F, Shaha A, Fish S, Tuttle M. Initial therapy with either thyroid lobectomy or total thyroidectomy without radioactive iodine remnant ablation is associated with very low rates of structural disease recurrence in properly selected patients with differentiated thyroid cancer. Clinical Endocrinology.2011;75:112-9. [ Links ]
17. Ullmann T, Gray K, Moore M, Zarnegar R, Fahey T. Current controversies in low-risk differentiated thyroid cancer: reducing over treatment in an era of over diagnosis. GlandSurg.2018;7:473-86. [ Links ]
18. Haugen B, Alexander E, Bible K, Doherty G, Mandel S, Nikiforov Y, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1-133. [ Links ]
19. Rajjoub S, Yan H, Calcatera N, Kuchta K, Wang C, Lutfi W, et al. Thyroid lobectomy is not sufficient for T2 papillary thyroid cancers. Surgery. 2018; 163:1134-43. [ Links ]
20. Craig S, Bysice A, Nakoneshny S, Pasieka J, Chandarana S. The identification of intraoperative risk factors can reduce, but not exclude, the need for completion thyroidectomy in low-risk papillary thyroid cancer patients. Thyroid. 2020;30:222-8. [ Links ]
21. Cranshaw I, Carnaille B. Micrometastases in thyroid cancer. An important finding? Surg Oncol. 2008;17:253-8. [ Links ]
22. Diker-Cohen T, Hirch D, Dhimon I, Bachar G, Akirov A, Duskin-Bitan H, et al. Impact of minimal extra-thyroid extension in differentiated thyroid cancer: Systematic Review and Metaanalysis. J Clin Endocrinol Metab. 2018;103:2100-6. [ Links ]
23. Shaha A, Migliacci J, Nixon I, Wang L, Wong R, Morris L, et al. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery. 2019;165:6-11. [ Links ]
24. Kim H, Sohn S, Jang H, Kim S, Chung J. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013;37:376-84. [ Links ]
25. Wang F, Yu X, Shen X, Zhu G, Huang Y, Liu R, et al. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocr Metabol. 2019;102:3241-50. [ Links ]
26. Joseph K, Edirimanne S, Eslick G. Multifocaliry as a prognostic factor in thyroid cancer: A meta-analysis. Int J Surg. 2018;50:121-5. [ Links ]
27. Harries V, Wang L, McGill M, Xu B, Tuttle R, Wong R, et al. Should multifocality be an indication for completion thyroidectomy in papillary thyroid carcinoma? Surgery. 2020;167:10-7. [ Links ]
28. DiMarco A, Wong M, Jayasekara J, Cole-Clark D, Aniss A, Glover A, et al. Risk of needing completion thyroidectomy for low-risk papillary thyroid cancers treated by lobectomy. BJS Open. 2019;3:299-304. [ Links ]
29. Lloyd R, Osamura R, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs, 4th ed. Lyon: IARC; 2017. [ Links ]
30. Ganly I, Ibrahimpasic T, Rivera M, Nixon I, Palmer F, Patel S, et al. Prognostic implications of papillary thyroid carcinoma with tall cell features. Thyroid. 2014;24:662-70. [ Links ]
31. Raffaelli M, Sessa L, De Crea C, Fadda G, Princi P, Rossi E, et al. Is it possibly to intraoperatively modulate the extend of thyroidectomy in small papillary thyroid carcinoma? Surgery. 2021;169:77-81. [ Links ]
32. Anuwong A. Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg. 2016;40:491-7. [ Links ]
33. Ahn J, Yi J. Transoral endoscopic thyroidectomy for thyroid carcinoma: outcomes and surgical completeness in 150 single-surgeon cases. Surg Endosc. 2020;34:861-7. [ Links ]
34. Chen Z, Song Y, Chen J, Zhang X, Pang F, Lin Z, et al. Safety and feasibility of the transoral endoscopic thyroidectomy vestibular approach with neuroprotection techniques for papillary thyroid carcinoma. BMC Surg. 2022;22:270. [ Links ]
35. Yi J, Yoob S, Kim H, Yu H, Kim S, Chai Y, et al. Transoral endoscopic surgery for papillary thyroid carcinoma: initial experience of a single surgeon in South Korea. Ann Surg Treat Res. 2018;95:73-5. [ Links ]
36. Voogd AI, Begueri Buquet AM, Valdez P, Russie G, Matsuda ME, Guerrisi J y cols. Tiroidectomía y paratiroidectomía endoscópica transoral con abordaje vestibular (TOETVA): Experiencia inicial en el Hospital Universitario Austral. Rev Argent Cirug 2021;113(2):205-15. [ Links ]
Received: October 31, 2023; Accepted: March 21, 2024











 texto en
texto en 



