Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cirugía
versión impresa ISSN 2250-639Xversión On-line ISSN 2250-639X
Rev. argent. cir. vol.116 no.2 Cap. Fed. jun. 2024 Epub 01-Jun-2024
http://dx.doi.org/10.25132/raac.v116.n2.1678
Original article
Microsurgery for phallic reconstruction
1Sector Paredes abdominales y microcirugía reconstructiva, Servicio de Cirugía General. Hospital Italiano de Buenos Aires Argentina
2Servicio de Urología, Hospital Italiano de Buenos Aires Argentina.
Background:
The absence of the penis causes serious physical limitations and psychosocial distress. Phallic reconstruction (PR) is a complex procedure because it involves the restoration of morphological and functional aspects. The radial forearm flap (RFF) is one of the best options for PR.
Objective:
The aim of this study was to describe the results of a series of patients undergoing PR using RFF.
Material and methods:
We conducted a descriptive observational study by reviewing the records of patients who underwent PR with RFF not related to gender dysphoria between July 2014 and July 2017.
Results:
Three patients aged 27, 36, and 22 years underwent reconstruction for traumatic amputation, oncologic treatment sequelae, and congenital defect, respectively. None of the patients developed flap necrosis. Somatic sensation was effective in all three cases and erogenous sensation in one. All patients reported overall satisfaction with the procedure.
Conclusion:
The use of RFF for PR by a multidisciplinary team produced satisfactory and uncomplicated results. Urethral reconstruction should be decided depending on each patient.
Keywords: penile defect; epispadias; phalloplasty; microsurgical free flap; forearm free flap
Introduction
The total or partial absence of the penis causes physical limitations and psychosocial distress. The impact of penile absence depends on the cause and the age of the patient1.
Phallic reconstruction (PR) may be needed due to congenital defects (hypospadias, epispadias or penile agenesis), cancer, trauma2, or gender dysphoria3.
It is a complex surgical procedure that must address morphological aspects such as size and shape, and functional aspects such as micturition, erection, ejaculation, and somatic and erogenous sensation3,4.
Due to its complexity, patients should undergo multidisciplinary evaluation, and surgery should be performed by a surgical team consisting of urologists and reconstructive surgeons specialized in microsurgery1,4.
The aim of this study was to describe the results of a series of patients undergoing microsurgical PR using radial forearm flap (RFF). We also evaluated the recovery of tactile and erogenous sensation, sexual function, and patient satisfaction using a non-validated scale.
Material and methods
Between July 2014 and July 2017, a team of specialists from the Departments of Urology and General Surgery performed PR using RFF on three patients without gender dysphoria.
Surgical technique
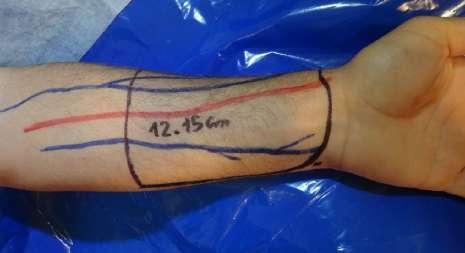
Outline and dissection of the radial forearm flap
In all patients, the non-dominant arm was used to raise the flap. Allen’s test was performed to evaluate if the circulation of the palm was adequate1.
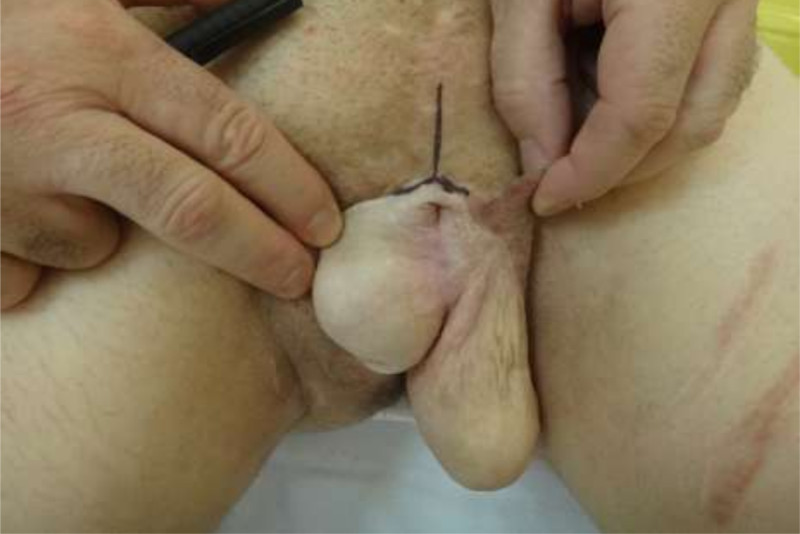
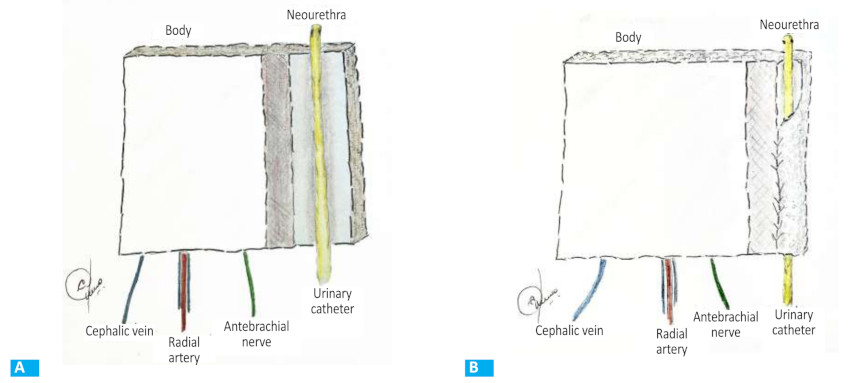
The flap was outlined over the volar aspect of the forearm and the course of the cephalic and basilic veins and the radial artery were marked. A skin paddle of 12 × 15 cm was outlined around the radial artery axis. In none of the cases was the flap intended for urethral reconstruction (Fig. 1). The flap was raised from distal to proximal, ligating the superficial peripheral veins dissecting the antebrachial fascia which was raised and included into the flap. The distal radial artery was then ligated, and the dissection was continued between the vascular bundle and the muscular plane that covers the radius. After reaching the proximal border of the flap, dissection continued following the course of the radial artery and superficial veins up to its origin in the brachial artery. In all three cases, the flap was raised including a superficial branch of the musculocutaneous sensory nerve.
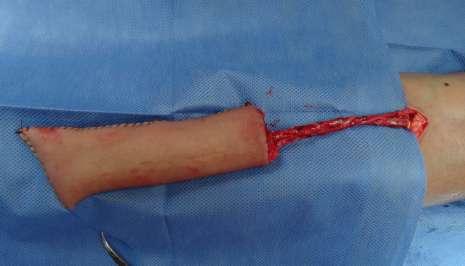
After the dissection was complete, the lateral edges of the flap were sutured, and the distal edge was closed to form a tube (Fig. 2). The radial artery and veins were sectioned after the recipient vessels were prepared for microsurgical anastomoses.
Dissection of vessels and recipient site
The urologists dissected the urethra and soft tissues. The team performing microsurgery accessed the recipient vessels in the epigastric region by making a right inguinal incision and opening the transversalis fascia to reach the preperitoneal space. In one patient the inguinal incision was extended to reach the femoral vessels. The radial artery was sectioned and the flap was sutured in the genital area. Its pedicle was then approached to the recipient vessels through a subcutaneous tunnel. Microarterial anastomoses were performed with separate sutures using a 9-0 nylon suture. An end-to-end anastomosis between the epigastric artery and the radial artery was performed in 2 cases, and an end-to-side anastomosis between the femoral artery and the radial artery was performed in 1 case. The same technique was used to perform venous anastomoses. Two venous anastomoses were performed in each of the three cases. Then an end-to-end anastomosis was made between the musculocutaneous nerve and the iliohypogastric nerve.
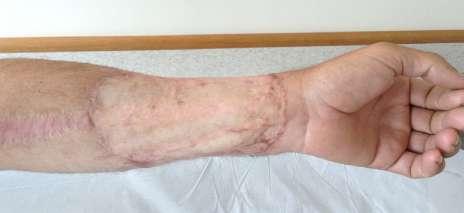
The donor site area was repaired with a thin skin graft from the thigh (Fig. 3).
Additional interventions
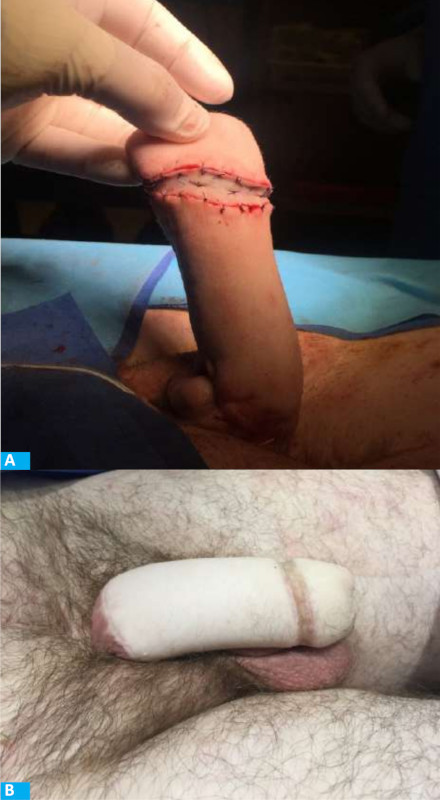
In the late postoperative period, additional procedures were performed to improve the appearance of the neophallus, such as the reconstruction of the coronal sulcus using the Norfolk technique1 (Fig. 4), creation of a neourethra to micturate in the standing position (Fig. 5) and insertion of a prosthesis to achieve erection.
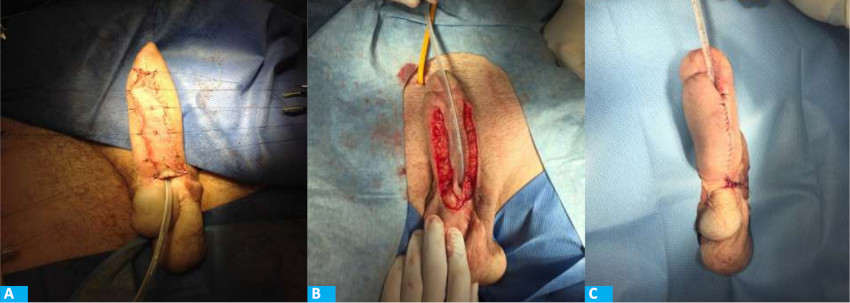
FIGURE 5 Delayed urethral reconstruction using skin graft. A: Thin skin graft in the ventral aspect of the neophallus. B: Creation of the urethra around a Silastic Foley catheter. C: Closure of the neourethra
The patients were asked to report on the tactile and erogenous sensation and the appearance of the neophallus at 12 and 24 months. Superficial sensation was assessed by physical examination, and erogenous sensation was only assessed in the patient with epispadias. The overall satisfaction with the procedure was evaluated by means of a non-validated questionnaire.
Results
The operative time ranged between 420 and 540 minutes and the patients were hospitalized for 4 days. The healing process for the forearm skin graft was completed in 36 to 45 days. There were no cases of partial or total flap necrosis of systemic complications.
Patient #1
A 27-year-old male presented total amputation of the penis, urethra and complete avulsion of the scrotal skin due to farming equipment accident2. The testes were preserved. Phallic reconstruction was scheduled 15 months after the accident (Fig. 6).
The urethra was connected to the base of the phallus for future staged urethroplasty.
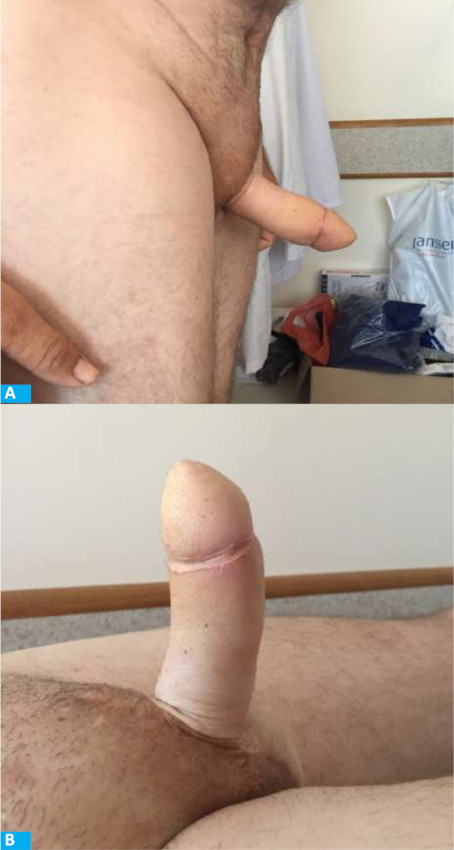
After 4 months, the coronal sulcus was defined1,5. After 8 months, a penile prosthesis was implanted for penetration (Fig. 7). After 12 months, a staged urethroplasty with skin graft was performed to bring the meatus to the coronal sulcus (Fig. 5). Six years later, the patient reports good flap sensation, adequate penetration during intercourse, and satisfaction with the procedure.
Patient #2
A 36-year-old male was operated on for a urethral tumor requiring resection of the urethra and corpora cavernosa6 (Fig. 8), chemotherapy and radiation therapy in the perineum6. Microvascular anastomoses were performed as in case #1. We did not perform neourethral reconstruction due to the distance of the perineal meatus from the base of the phallus and the presence of the irradiated perineum.
After 4 months, the coronal sulcus was defined. Four years later, the patient reports good flap sensation and is waiting for penile implant.
Patient #3
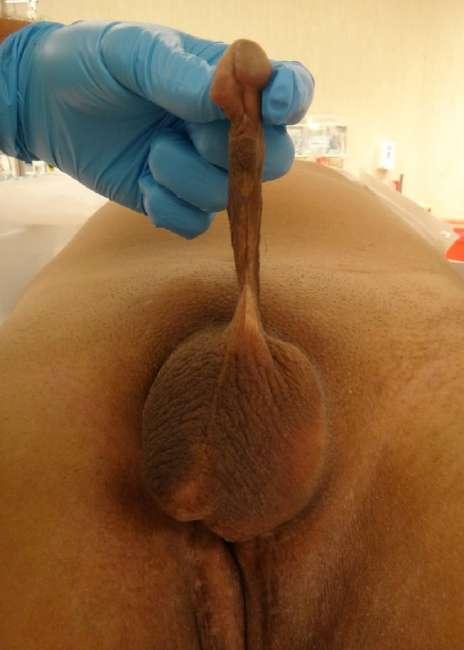
A 22-year-old patient underwent multiple surgeries for epispadias and congenital bladder exstrophy7. On physical examination, the penis measured 4 cm in length and the glans had preserved erogenous sensation. The bladder was emptied through intermittent clean catheterization via the urethra (Fig. 9).

FIGURE 9 Patient with epispadias, bladder exstrophy and micropenis with preserved erogenous sensation
An end-to-side arterial microvascular anastomosis was performed between the radial artery and the right femoral artery, and an end-to-side venous microvascular anastomosis was performed between the superficial cephalic vein of the flap and the right femoral vein.
The glans was preserved and sutured to the base of the neophallus to preserve erogenous sensation (Fig. 10). The urethra was dissected from the patient’s penis and brought to the perineum for intermittent clean catheterization.
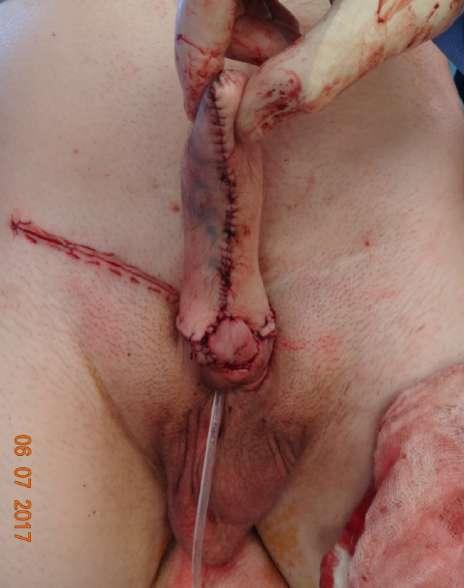
FIGURE 10 The micropenis was preserved and sutured to the base of the neophallus to preserve erogenous sensation
After 4 months, the coronal sulcus was defined. Four years later, the patient reports good flap sensation and preserved erogenous sensation of the glans and is awaiting a penile implant.
Discussion
The first reconstruction of the penis was performed by Bograz5 in 1936, who constructed a tubular flap with skin taken from the abdomen and did not reconstruct the urethra. Later, Maltz and subsequently Gillies and Harrison added the tube-within-a-tube concept to reconstruct the neourethra5 (Fig. 11). Thereafter, some attempts were made with musculocutaneous flaps from the thigh5.
A new stage began with the development of microsurgery. Originally described in 1982 by Song et al.8, the radial forearm free flap was first reported for penile reconstruction in 1984 by Chang and Hwang9 who used this flap in seven patients with total penile amputation. After this publication, many authors adopted this type of flap3,4,6.
The penis has multiple functions and to achieve all of them in a single operation is a very complex task1,7,10.
The goal of PR is to restore the patient’s body image, enable micturition while standing, and allow for penetrative sexual intercourse.
In the case of adults who have lost their normal phallus, it is very difficult for them to regain what they already knew. This is different in children, as they have grown up without a phallus and lack a body image with a penis, which creates fewer expectations7.
In a systematic review and meta-analysis published by Wang et al11 in 2022, of 1731 patients undergoing PR, radial flap was the most used (75.1%). This is consistent with the meta-analysis published by Yao et al. in 201812.
The radial flap is considered the best option for PR1,3,4,13: it is safe, quick to raise, malleable, allows the dissection of wide skin paddles and the repair of complex structures such as the urethra with the tube-in-tube technique1 (Fig. 11). Its vascular pedicle y long and its artery has a diameter of 3 mm.
As it has several veins, it allows double venous drainage. It is innervated by the lateral antebrachial cutaneous nerve, which provides sensation to the flap.
Two surgical teams can work simultaneously. The donor area requires skin grafting and leaves aesthetic but not functional sequelae.
With this flap, the aesthetic results are acceptable, but should be improved with additional surgeries1,4. The reconstruction of the coronal sulcus and glans are essential components of a complete PR. Coronoplasty using the Norfolk1 technique creates a prominent and well-marked coronal sulcus separating the glans from the body of the phallus (Fig. 4)
Urethral reconstruction allows micturition through the neophallus and in the standing position. The neourethra can be created simultaneously when the radial flap is designed or can be delayed to another stage using skin grafts4. Some authors prefer this latter option because it has fewer complications4, Simultaneous reconstruction is associated with high rates of fistulas (12%) and strictures (35%)3,4,10; therefore, urethral reconstruction should be decided depending on each patient.
We did not perform simultaneous urethral reconstruction in our cases. The patient with epispadias has been micturating since childhood by intermittent clean catheterization through a penoscrotal meatus.
The patient with cancer micturated through a very proximal perineal meatus due to oncologic resection; his perineum had surgical scars and had been irradiated6.
We performed a delayed urethral reconstruction in the patient with traumatic amputation. In this case, we consider it feasible and necessary because the patient works in the countryside and micturition while standing makes his work easier.
Erection is achieved by implanting a penile prosthesis4. The complications of penile prosthesis implantation in these patients are greater than in patients with normal penises10.
Finally, we believe that phallic reconstruction plays a very important role in patients without gender dysphoria, allowing them to reintegrate into society, which would be very serious problem without this surgery1,4, 5, 7,10,13.
In conclusion, the use of RFF for PR by a multidisciplinary team produced satisfactory and uncomplicated results.
Other additional procedures are necessary to achieve better aesthetic and functional results. Urethral reconstruction should be decided depending on each patient.
Referencias bibliográficas /References
1. Gilbert DA, Winslow BH, Gilbert DM, Jordan GH, Horton CE. Transsexual surgery in the genetic female. Clin Plast Surg. 1988;15:471-8. [ Links ]
2. De Luca FG, Garaffa G, Maurizi A, Manzi E, De Dominicis C, Ralph D. Total phallic reconstruction after penile amputation for donkey bite: case report and review of the literature. Arch Ital Urol Androl. 2017;89 (2): 166-8. [ Links ]
3. Morrison SD, Shakir A, Vyas KS, Kirby J, Crane CN, Lee GK. Phalloplasty: A review of techniques and outcomes. Plast Reconst Surg. 2016;138:594-615. [ Links ]
4. Garaffa G, Spilotros M, Christopher NA, Ralph D J. Total Phallic Reconstruction Using Radial Artery Based Forearm Free Flap Phalloplasty in Patients with Epispadias-Exstrophy Complex. J Urol. 2014;192:814-20. [ Links ]
5. Garaffa G, Christopher NA, Ralph DJ. Total phallic reconstruction in female-to-male transsexuals. Eur Urol. 2010;57:715-22. [ Links ]
6. Garaffa G, Raheem A, Christopher N, Ralph D. Total phallic reconstruction after penile amputation for carcinoma. BJU Int. 2009;104:852-6. [ Links ]
7. Massanyi EZ, Gupta A, Goel S, Gearhart JP, Burnett AL, Bivalacqua TJ, et al. Radial forearm free flap phalloplasty for penile inadequacy in patients with extrophy. J Urol. 2013;190: 1577-82. [ Links ]
8. Song R, Gao Y, Song Y, Yu Y, Song Y. The forearm flap. Clin Plast Surg. 1982;9:21-6. [ Links ]
9. Chang T, Hwang W. Forearm flap in one-stage reconstruction of the penis. Plast Reconstr Surg. 1984;74:251-8. [ Links ]
10. Monstrey S, Hoebeke P, Dhont M, Selvaggi G, Hamdi M, Van Landuyt K, et al. Radial forearm phalloplasty: a review of 81 cases. Eur J Plast Surg. 2005;28:206-12. [ Links ]
11. Wang AMQ, Tsang V, Mankowski P, Demsey D, Kavanagh A, Genoway K. Outcomes Following Gender Affirming Phalloplasty: A Systematic Review and Meta-Analysis. Sex Med Rev. 2022;10(4):499-512. [ Links ]
12. Yao A, Ingargiola MJ, López CD, Sanati-Mehrizy P, Burish NM, Jablonka EM, et al. Total penile reconstruction: a systematic review. J Plast Reconstr Aesthet Surg. 2018;71(6):788-806. [ Links ]
13. Heston A, Esmonde N, Dugi DD, Berli JU. Phalloplasty: techniques and outcomes. Transl Androl Urol. 2019; 8(3):254-65. [ Links ]
Received: September 05, 2023; Accepted: January 19, 2024











 texto en
texto en