Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista agronómica del noroeste argentino
versión impresa ISSN 0080-2069versión On-line ISSN 2314-369X
Rev. agron. noroeste arg. vol.38 no.1 San Miguel de Tucumán jun. 2018
SCIENTIFIC ARTICLE
Performance of petunia crop in response to inoculation with Azospirillum brasilense
Desempeño del cultivo de petunia en respuesta a la inoculación con Azospirillum brasilense
L.M. Toffoli1; N.N. Medrano1; M.G. Martínez-Zamora2; M.F. Guerrero-Molina2; R.O. Pedraza3; S.M. Salazar1,3*
1Estación Experimental Agropecuaria Famaillá, Instituto Nacional de Tecnología Agropecuaria. Ruta Provincial 301 Km 32, (4132), Famaillá, Tucumán, Argentina.
2Instituto Superior de Investigaciones Biológicas (INSIBIO-UNT-CONICET), Instituto de Química Biológica “Dr. Bernabé Bloj”, Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán. Chacabuco 461 (4000), San Miguel de Tucumán, Tucumán, Argentina.
3Facultad de Agronomía y Zootecnia. Universidad Nacional de Tucumán, Avda. Kirchner 1900, (4000), San Miguel de Tucumán, Tucumán, Argentina. *E-mail: salazar.sergio@inta.gob.ar
Abstract
The present study was carried out to analyze the performance of Petunia hybrida when inoculated with Azospirillum brasilense REC3. Parameters evaluated were root length, dry weight of roots and shoots, leaf area, leaf greenness, precocity (days to flowering) and number of flowers in plants. Azospirillum inoculation increased root growth, shoot weight and number of flowers compared to controls. Time to flowering, a fundamental feature from the economic point of view, was shortened in inoculated plants. These findings indicated that inoculation with A. brasilense strain REC3 resulted in an increase in vegetative growth and an early and profuse flowering, thus reducing fertilization costs and environmental pollution.
Keywords: Petunia hybrida;Biofertilization; PGPR; Flowering; Yield.
Resumen
El presente estudio se llevó a cabo para analizar el comportamiento de Petunia hybrida en respuesta a la inoculación con Azospirillum brasilense REC3. Los parámetros evaluados fueron: longitud de raíz, peso seco de raíces y tallos, área foliar, verdor de las hojas, precocidad (días hasta floración) y número de flores. La inoculación con Azospirillum incrementó el crecimiento radicular, el peso de los tallos y el número de flores comparando con los controles. El tiempo transcurrido hasta la floración, rasgo fundamental desde el punto de vista económico, disminuyó en plantas inoculadas. Estos hallazgos indican que la inoculación con A. brasilense REC3 contribuye a incrementar el crecimiento vegetativo y a obtener una floración más temprana y abundante, por lo que podrían así reducirse los costos de producción y la contaminación ambiental.
Palabras clave: Petunia hybrida;Biofertilización; PGPR; Floración; Rendimiento.
Received 04/24/18; Accepted 06/14/18.
The authors declare to have no conflict of interest.
Introduction
Floriculture is an intensive agricultural activity that covers the cultivation of ornamental flowers and plants for many purposes, including cultures of cut flowers and seedling production (da Silva Vieira et al., 2010).
Petunia (Petunia x hybrida) derives from P. integrifolia and P. axillaris,two of many Petunia species endemic to South America (Stehmann et al., 2009). Because of its particular traits, e.g. versatility, long flowering season, and wide availability of colors and shapes resulting from continuous genetic breeding, this flower crop is one of the main annual flowering plants worldwide.
Increased flower production, quality of flowers and perfection in plant shape are important objectives in bedding and flower production (El-Mokadem and Mona, 2014). Great amounts of chemical fertilizers are needed to reach these goals, which impacts directly on production costs, while also having negative and unpredictable effects on the environment, and causing serious risks to human and animal health (Pedraza et al., 2007).
The current tendency is to reduce the use of fertilizers, with biofertilization being one of the most valid alternatives (Fuentes-Ramírez and Caballero-Mellado, 2006). In this sense, plant growth-promoting rhizobacteria (PGPR) represent a wide variety of soil bacterial genera with a capacity of stimulating plant growth (Fuentes-Ramírez and Caballero-Mellado, 2006; Bashan and de-Bashan, 2010).
Azospirillum spp.is a free-living rhizobacterium that belongs to this group, and which is capable of affecting the growth and yield of numerous plant species, many of which are of agronomical and ecological importance (Bashan and de-Bashan, 2010). Azospirillum brasilense promotes plant growth through several mechanisms activated simultaneously or sequentially (Bashan and de-Bashan, 2010), like N2 fixation, phosphate solubilization, siderophore production (Pedraza et al., 2010), and direct and indirect control of numerous phytopathogens (Bashan and de-Bashan, 2010). Nevertheless, the main hypothesis about its growth promoting capacity points at its apparent ability to produce various phytohormones like auxins, which lead to improvements in root growth and a better water and mineral absorption, which in turn result in larger, and in many cases, more productive plants (Pedraza et al., 2007; Bashan and de-Bashan, 2010). Even when there are many works describing the growth promoting effect of Azospirillum in agronomically important crops, there are few reports regarding the use of PGPR as bioinoculants in ornamental crops (Mostafa and Abo-Baker, 2010; El-Mokadem and Mona, 2014). Azospirillum brasilense REC3 possesses desirable traits as a PGPR, such as its capacity to fix nitrogen, produce siderophores and indoles, and promote plant growth in strawberry (Pedraza et al., 2010; Lovaisa et al., 2015). This strain was isolated and characterized by Pedraza et al. (2007) from surface sterilized strawberry roots.
Considering such background, the aim of this work was to evaluate petunia growth and flowering in response to inoculation with strain REC3 of A. brasilense as a bioinoculant.
Materials and methods
Plant material and growth conditions
One-month-old seedlings of Petunia x hybrida UltraTM were obtained at a commercial nursery garden in Buenos Aires, Argentina. The experiment was conducted from April to June 2013, in a greenhouse belonging to the INTA Famaillá Experimental Station, in the province of Tucumán, Argentina (Latitude: 27º03’S; 65º25’W, 363 m).
Bacterial growth condition, inoculation and fertilization
Experiments were randomized and had three treatments: i) REC3 inoculated plants; ii) plants fertilized with chemical products at recommended doses as chemical control plants; iii) non-inoculated and non-fertilized as absolute control plants. For plant inoculation, a bacterial inoculum of strain REC3 was prepared on N2-free malate liquid medium (NFb), supplemented with 1 % NH4Cl (w/v) and incubated during 72 h at 30 ºC. The cultures were centrifuged at 7,000 g for 10 minutes and washed twice with buffer phosphate, pH 7.0, to remove any culture medium residue that may interfere with the experiment. Petunia plantlets were inoculated by submerging their roots in the bacterial suspension (106 UFC mL-1) for 30 minutes, and then transferred to 900 mL containers with sterile soil. Roots of control plants (with or without chemical fertilization) were dipped in water for 30 min before transplanting them. In the case of chemically fertilized control plants, recommended doses for fertilization were 1 g/L of 19:19:19 NPK a week after transplanting, and 1 g/L of KNO3 once a week.
Data collection
To evaluate the growth-promoting effect of bacterial inoculation, destructive and non-destructive parameters were measured 15, 30, 45 and 60 days after treatment (DAT). Destructive parameters were root length (cm), fresh and dry weight of roots and shoots (g), and leaf area (cm2). Leaf greenness (SPAD index), a non-destructive parameter, was determined with a Minolta SPAD-502 meter. In addition, the number of days from transplanting to flowering and the number of flowers per plant were counted once a week.
Data analysis
For each treatment, 20 repetitions for non-destructive parameters and 5 repetitions for destructive parameters were evaluated. Results were analyzed with InfoStat version 2017 (Di Rienzo et al., 2017). Root length, dry weight of roots and shoots, leaf area and SPAD index were analyzed by means of an analysis of variance (ANOVA) test. Significant differences between means were compared using the DGC comparison method at p < 0.05. To evaluate days until flowering and number of flowers, a generalized linear model with Poisson distribution with logit link function was used.
Results and discussion
Although throughout the assay inoculated plants developed longer roots, significant differences were found 45 DAT between root length averages in inoculated plants (32.4 ± 2.67 cm) and conventionally fertilized plants (25.4 ± 2.67 cm; Table 1). Also, plants of comparable root length showed a greater dry weight in the case of inoculated plants, probably due to root area increase, except 30 DAT. Average dry weight of shoots was higher in A. brasilense inoculated plants compared to conventionally fertilized and control plants. The mean value of shoot dry weight of inoculated plants (0.12 ± 0.01 g) was significantly different from those of fertilized (0.08 ± 0.01 g) and control plants (0.08 ± 0.01 g) 15 DAT (Table 1).
Regardless of the treatment, leaf greenness (SPAD index) increased between 15 and 30 DAT and decreased between 30 and 60 DAT (Figure 1). Between 15 and 30 DAT, greenness index in Azospirillum inoculated plants was higher than those in conventionally fertilized and control plants, with a significant difference (p= 0.0033) between inoculated and conventionally fertilized plants at 15 DAT (32.94 ± 0.43 and 30.99 ± 0.43, respectively; Figure 1).

Figure 1. Greenness index measured in Azospirillum inoculated (Az), conventionally fertilized (Ft), and control (Ct) plants, 15, 30, 45 and 60 days after treatment (DAT). Different letters indicate significant differences at p < 0.05.
Days to flowering per plant (number of days from transplanting to flowering) are shown in Figure 2. We observed earlier flowering in inoculated plants when compared to conventionally fertilized plants (28 ±1.19 and 32 ± 1.26 days to flowering, respectively, with p= 0.0001).
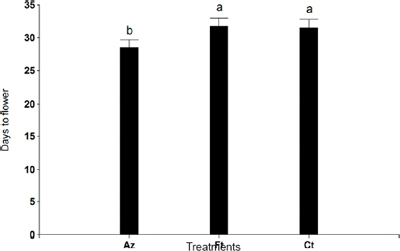
Figure 2. Days to flowering measured in Azospirillum inoculated (Az), conventionally fertilized (Ft), and control (Ct) plants. Different letters indicate significant differences at p < 0.05.
Results showed that throughout the assay, the number of flowers in Azospirillum inoculated Petunia x hybrida was higher than in conventionally fertilized plants, and significantly (p = 0.0001) higher than in control plants (Figure 3).
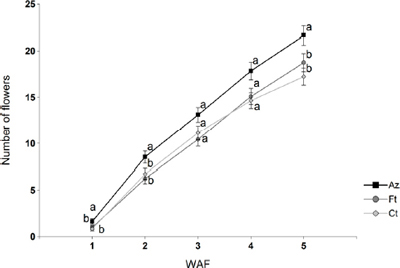
Figure 3. Number of flowers per plant counted in Azospirillum inoculated (Az), conventionally fertilized (Ft), and control (Ct) plants, every week after flowering (WAF). Different letters indicate significant differences at p < 0.05.
The use of Azospirillum as a plant growth promoter is well documented in agronomically important crops (Bashan and de-Bashan, 2010), but there are few reports related to the use of PGPR as bioinoculants in ornamental crops to reduce fertilizer applications that may lead to environmental pollution (El-Mokadem and Mona, 2014).
Inoculation of plants with Azospirillum brasilense REC3 led to increased root growth compared with fertilized plants. Our results are in agreenment with those reported by Pedraza et al. (2010), who observed that the root length and dry weight of roots and shoots of REC3-inoculated strawberry plants were superior than those of uninoculated plants. Guerrero-Molina et al. (2014) also demonstrated that inoculating strawberry plants with REC3 increased the number of lateral roots and root hairs. Larger root systems with a greater surface area might result in an increased uptake of nutrients and water, and hence a further development of plant aerial parts, as observed in petunia plants.
Bacterial inoculation increases chlorophyll (Chl) content, which might be due to the fact that it secures a higher uptake of N2, either because of the ability of the bacterial strain to fix atmospheric nitrogen or because of the improvement of the radicular system, or both. In previous studies, Pedraza et al. (2007) demonstrated the potential N2-fixing capacity of local bacterial isolates, including strain REC3, using molecular approaches. In addition, Guerrero-Molina et al. (2014) found higher nitrogen content in leaves of REC3-inoculated plants of strawberry when contrasting them with those of control plants. Recent research indicates a close link between leaf Chl concentration and leaf N2 content, which makes sense because most leaf N2 is found in Chl molecules (Boussadia et al., 2011).
An anticipated flowering might be attributed to the early development of plants with an efficient nutrient and water uptake, which results from inoculation. Optimal growth during the first month is essential for a good establishment in a greenhouse, and shortening the time required for plant production counts as an advantage. Our results are in agreement with those from Mostafa and Abo-Baker (2010), who reported an earlier flowering in plants inoculated with PGPR compared to the control. Similarly, Mia et al. (2010) managed to anticipate flowering when inoculating banana with strain Sp7 of A. brasilense. Ali et al. (2014) found an early flowering in Gladiolus grandiflorus plants inoculated with Azospirillum sp.The increment of flower number in inoculated plants can be attributed to an improved nutrient uptake, with a major vegetative growth, which causes plants to flower more profusely. Our findings are consistent with those cited for other species in previous studies, which stated that the number of flowers produced by a plant is proportional to plant nutrient content (Sardans et al., 2005).
Conclusion
Based on our results, it can be concluded that inoculation with Azospirillum brasilense strain REC3 contributed to the increase in vegetative growth and an early flowering in petunia. As a consequence, labor and fertilization costs could be reduced and environmental impact could be diminished.
Acknowledgements
This study is part of a joint project between INTA-Famaillá and Facultad de Agronomía y Zootecnia. This work was supported by Instituto Nacional de Tecnología Agropecuaria (INTA) Program PNHFA 1106093 and TUSGO 1231101, andConsejo de Investigación de la Universidad Nacional de Tucumán (PIUNT) Program 26/A401. MGMZ and MFGM are members of CONICET.
References
1. Ali A., Mehmood T., Hussain R., Bashir A., Raza S., Najam-ud-Din, Ahmad A. (2014). Investigation of biofertilizer influence on vegetative growth, flower quality, bulb yield and nutrient uptake in gladiolus (Gladiolus grandiflorus L.). International Journal of Plant, Animal and Environmental Sciences 4 (1): 94-99. [ Links ]
2. Bashan Y., De-Bashan L.E. (2010). How the plant growth-promoting bacterium Azospirillum promotes plant growth- a critical assessment. Advances in Agronomy 108: 77-136. [ Links ]
3. Boussadia O., Steppe K., Zgallai H., Ben El Hadj S., Lemuer R., Van Labeke M.C. (2011). Nondestructive determination of nitrogen and chlorophyll content in olive tree leaves and the relation with photosynthesis and fluorescence parameters. Photosynthetica 49: 149-153. [ Links ]
4. da Silva Vieira M.R., Citadini V., Pereira Lima G.C., De Souza A.V., De Souza Alves L. (2010). Use of gibberellin in floriculture. African Journal of Biotechnology 9: 9118-9121. [ Links ]
5. Di Rienzo J.A., Casanoves F., Balzarini, M.G., Gonzalez L., Tablada M., Robledo C.W. (2017). InfoStat versión 2017. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL: http://www.infostat.com.ar [ Links ]
6. El-Mokadem H.E., Mona S. (2014). Effect of bio and chemical fertilizers on growth and flowering of Petunia hybrida plants. American Journal of Plant Physiology 9 (2): 68-77. [ Links ]
7. Fuentes-Ramírez L.E., Caballero-Mellado J. (2006). Bacterial Biofertilizers. In: PGPR: Biocontrol and Biofertilization. Sidiqui Z.A. (Ed). Springer, Netherlands. Pp. 143-172. [ Links ]
8. Guerrero-Molina M.F., Lovaisa N.C., Salazar S.M., Díaz-Ricci J.C., Pedraza R.O. (2014). Elemental composition of strawberry plants inoculated with the plant growth- promoting bacterium Azospirillum brasilense REC3 assessed by scanning electron microscopy and energy dispersive X-ray analysis. Plant Biology 16: 726-731. [ Links ]
9. Lovaisa N.C., Guerrero-Molina M.F., Delaporte Quintana P.G.A., Salazar S.M. (2015). Response of strawberry plants inoculated with Azospirillum and Burkholderia at field conditions. Revista Agronómica del Noroeste Argentino 35 (1): 33-36. [ Links ]
10. Mia M.A.B., Shamsuddin Z.H., Mahmood M. (2010). Use of plant growth promoting bacteria in banana: a new insight for sustainable banana production. International Journal Agriculture and Biology 12: 459-467. [ Links ]
11. Mostafa G.G., Abo-Baker A.A. (2010). Effect of bio-and chemical fertilization on growth of sunflower (Helianthus annuus L.) at south valley area. Asian Journal of Crop Science 2: 137-146. [ Links ]
12. Pedraza R.O., Motok J., Salazar S.M., Ragout A.L., Mentel M.I., Tortora M.L., Guerrero-Molina M.F., Winik B.C., Díaz-Ricci J.C. (2010). Growth-promotion of strawberry plants inoculated with Azospirillum brasilense. World Journal of Microbiology and Biotechnology 26: 265-272. [ Links ]
13. Pedraza R.O., Motok J., Tortora M.L., Salazar S.M., Díaz-Ricci J.C. (2007). Natural ocurrente of Azospirillum brasilense in strawberry plants. Plant and Soil 295: 169-178. [ Links ]
14. Sardans J., Roda F., Peñuelas J. (2005). Effect of water and nutrient pulse supply on Rosmarinus officinalis growth, nutrient content and flowering in field. Environmental and Experimental Botany 53: 1-11. [ Links ]
15. Stehmann J.R., Lorenz-Lemke A.P., Freitas L.B., Semir J. (2009). The Genus Petunia. In: Petunia. Gerats T., Strommer J. (Eds.). Springer, USA. Pp. 1-28. [ Links ]














