Introduction
Cut flower and flower bulb crops are some of the most profitable ones worldwide, and they constitute the main agricultural activity in several countries (Morisigue et al., 2012). Among cut flower species, freesias (Freesia x hybrida) rank high in the international flower market as they lead to soaring annual profits for countries that produce this flower (Berecici and Bala, 2011; Bazaraa, 2018).
Freesias are monocotyledons and herbaceous plants, which originally come from Cape Province, South Africa (Africa) (Wang, 2007). They are geophytes that belong to the Iridaceae family, and like saffron (Crocus sativus L.) and gladioli (Gladiolus grandiflorus L.), they produce characteristically solid bulbs (corms) that are protected by the basal parts of dry leaves (the tunic), which overlap and come together on the top (Ahmad et al., 2011; Fatihullah and Bostan, 2018). These constitute the specialized underground vegetative structure that allows them to survive under adverse conditions, while also fulfilling the role of a vegetative propagation organ (Dole, 2003; Thompson et al., 2011).
Freesias have simple, ensiform, isolateral leaves which exhibit parallel venation, a prominent central vein, and sheathing leaf base.
The plant has a cymose inflorescence (cyme) on top of the flower scape, with 10 to 12 tubular, sessile, and fragrant simple or double flowers, each one having a pair of bracts underneath. The base of the inflorescence forms an angle of about 90° with the flower scape, so the latter has a horizontal orientation, and the flowers remain perpendicular to it (Wang, 2007).
The development of the crop is marked by a series of identifiable events that result in structural changes in the plant (Dambreville et al., 2015). These changes define crop development stages and are expressed as cell differentiation processes, organogenesis, morphogenesis, and senescence (Streck et al., 2012; Flores-Magdaleno et al., 2014).
Phenology studies and describes the changes that come about during plant development (Meier et al., 2009; Tang et al., 2016). The BBCH scale (Biologische Bundesanstalt, Bundessortenamt, Chemische Industrie) is used worldwide to describe crop development stages through a uniform coding system, based on the scale Zadoks et al. (1974) proposed for cereals (Chmielewski, 2003; Meier et al., 2009). Since the system is standardized, it enables the description of equivalent development stages of different crops and weeds, by means of the same code (Lancashire et al., 1991; Finn et al., 2007; Meier et al., 2009; Rajan et al., 2011 Javadzadeh, 2018).
The BBCH scale divides the growth cycle into 10 main phases, considering the main morphological changes that plants undergo. Moreover, the scale distinguishes secondary stages within the primary ones, based on other plant changes that are worth describing. Both references are used jointly to describe specific moments in crop development, which are thus named with a digit from 0 to 9, in ascending order. Therefore, the phenological scale is coded into two digits, with the first referring to the main stage, and the second indicating the secondary one (Meier et al., 2009).
However, on some occasions the phenological characterization of some species requires a further description and digit (mesostage). This again ranges from 0 to 9, and is included between the main stage and secondary stage digits (Meier et al., 2008; Meier et al., 2009; Martinelli and Galasso, 2011; Niemenak et al., 2010; Hernández Delgado et al., 2011; López-Bellido et al., 2016; Kishore, 2018).
It is necessary to learn about and characterize crop development phases with the help of an adequate phenological scale, so as to carry out crop management practices, such as plantation, irrigation, fertilization, or pest and disease management, at the optimum moment in the crop cycle. A scale is also essential for predicting crop development and harvesting dates (Chmielewski, 2003; Castro et al., 2018; Fagundes et al., 2010; Bouzo and Küchen, 2012; Vadez et al., 2013).
Given that no scale is currently used to describe the phenological events of Freesia x hybrida crop, this work aims to characterize and code them according to the general principles of the BBCH-scale.
Materials and methods
Laboratory and field trials were conducted in 2018, with the aim of describing and coding freesia crop phenology. The vegetable material used in the assays consisted of Freesia x hybrida cv. Ivonne® corms, with a 1.8 to 2.2 cm diameter. These were disinfected by submerging into an antifungal Folpet 80% WP solution, with a 1 g/l concentration.
Laboratory trial
The trial was conducted in the laboratory of the Cátedra de Plantas Ornamentales y Floricultura, of the Facultad de Agronomía y Zootecnia (Universidad Nacional de Tucumán, Tucumán, Argentina). Thirty corms were used, placing them in groups of 10 on 0.2 × 0.25 × 0.05 m plastic trays, with wet absorbent paper in the base. These were kept in the dark, under a 20 - 25 ºC temperature.
The changes that took place underground were observed, once corm dormancy was over. Daily photographic records were kept until the end of sprouting, which is when corms were finally discarded.
Field trial
This trial was conducted in an experimental field of Finca El Manantial, which belongs to Facultad de Agronomía y Zootecnia (Universidad Nacional de Tucumán, Tucumán, Argentina) (26º 50' 6.9'' S - 65º 16' 44.6'' W). The site is located in the subhumid-humid central plain region, which has a monsoon subhumid-humid subtropical climate with a dry season in winter. Average annual rainfall rates are higher than 950 mm, and mean annual temperature reaches 19.7°C. Frosts are scarce and have a low intensity (Torres Bruchmann, 1973).
Corms were planted in 4 l polyethylene containers, which were placed on iron counters outdoors. The substrate used in the trials consisted of soil and agricultural perlite 3:1 (v/v), with an electrical conductivity = 1.11 dS/m, pH = 6.4 and organic matter = 2.5-3.5%.
Four planting dates were considered so as to observe whether the phenological phases were actually reached under different weather conditions: February 28, March 21, April 11 and May 2. On each of these dates, three corms were planted 2 cm deep in the containers, each one of which was taken as the experimental unit. A total of 15 containers were evaluated per date (45 corms overall), five of which were allotted to destructive trials aimed at observing the development of planted corms, whereas the remaining 10 were monitored for changes in the aerial parts of the crop.
The plants were observed from plantation to senescence on a daily basis, so that precise phenological details could be recorded. Photographs of the plants were taken in each development phase with a Samsung® 14 mpx digital camera (PL120ZF, Samsung electronics CO.,LTD., China).
Each plant was considered as having reached emergence stage when the sprout had risen 1 cm above the ground. After emergence, the number of produced leaves was counted, taking each produced leaf as new when its blade was 2 cm long (as measured from the apex to its insertion into the false stem that is formed by the sheaths).
Complete inflorescence was taken as a visual sign that the reproductive phase had begun. In the freesia flower scape, it was possible to observe that the terminal inflorescence had developed, together with other inflorescences in ramifications of the scape. When examining each inflorescence, it was found that the rachis was bent exactly on the spot where the first flower was inserted, and that it had lengthened.
The inflorescence was divided into three parts (basal, medial, and distal) in order to describe flower development, tepal pigmentation, the separation of the flower from the bracts, and the visualization of the corolla tube.
The corms were characterized after extracting them from the plants, whenever a new leaf developed and when the spike emerged. The tunics were discarded, while taking care that the root system was not damaged. Observations were made to monitor the growth of the contractile root, the development of the new corm, and the process of dehydration of the mother corm.
In order to identify senescence, all the leaves in the plants were examined for changes in color, from green to brown.
The crop management practices followed during the trial were manual weeding, manual irrigation, tutoring of plants with individual stakes and chemical pest management with lambdacialotrine (400 cm3 / ha).
Results
In Table 1, the scale proposed for freesia is presented, showing its three-digit coding.
Six main development phases were recorded, described and coded for the freesia crop: sprouting (stage 0), leaf development (stage 1), development of vegetative propagated organ (stage 4), inflorescence emergence (stage 5), flowering (stage 6), and senescence and beginning of dormancy (stage 9) (Table 1).
Table 1 Description of the phenological stages observed in Freesia x hybrida coded according to the BBCH-scale.
| Code | Description |
|---|---|
| Main development stage 0: sprouting | |
| 000 | Dormant corm. |
| 003 | Presence of radicles at the corm base. |
| 005 | Emergence of fibrous roots and the sprout, which is protected by the tunic. |
| 007 | Increase in root and sprout length. |
| 009 | Emergence of the sprout above the ground |
| Main development stage 1: leaf development | |
| 101 | Appearance of first leaf blade (> 2 cm). |
| 102 | Appearance of second leaf blade (> 2 cm). |
| 103 | Appearance of third leaf blade (> 2 cm). |
| 1.. | Stages continue until… |
| 109 | Appearance of ninth leaf blade (> 2 cm). |
| 110 | Appearance of tenth leaf blade (> 2 cm). |
| 111 | Appearance of eleventh leaf blade (> 2 cm). |
| 112 | Appearance of twelfth leaf blade (> 2 cm). |
| 11.n | Stages continue until the appearance of n leaf blade (> 2 cm). |
| Main development stage 4: development of the vegetative propagated organ | |
| 400 | Development of the contractile root at the base of the leaf sheaths. |
| 403 | Beginning of the development of the new corm. Increase in contractile root length. |
| 405 | Increase in new corm diameter and root mass, beginning of dehydration of the mother corm. |
| 407 | Dehydrated mother corm. The new corm reaches its final size. Presence of cormels at the base. |
| 409 | Beginning of corm dormancy. Time for harvesting the organ. |
| Main development stage 5: inflorescence emergence | |
| 500 | Appearance of flag leaf. |
| 501 | Emergence of inflorescence. |
| 502 | Growth of the flower scape in the inflorescence. |
| 503 | Curvature of inflorescence at the base of the first flower. |
| 504 | Increase in rachis length, distancing of flowers. |
| 505 | Basal flowers, inside the bracts, closed and exhibiting color. |
| 506 | Medial flowers, inside the bracts, closed and exhibiting color. |
| 507 | Distal flowers, inside the bracts, closed and exhibiting color. |
| 508 | All the flowers are within the bracts, and they are closed and pigmented. |
| 509 | Basal flowers of the inflorescence separated from the bracts, corolla tube visible, tepals closed. Harvesting time. |
| 511 | Emergence of the second inflorescence (lateral). |
| 512 | Increase in length of the flower scape of the secondary inflorescence. |
| 513 | Curvature of the second inflorescence at the base of the first flower. |
| 51. | Stages continue until… |
| 519 | The basal flowers of the secondary inflorescence are separated from the bracts, and the corolla tube is visible, with closed tepals. Harvesting time. |
| 521 | Emergence of the third inflorescence. |
| 52. | Stages continue until… |
| 529 | The basal flowers of the third inflorescence are separated from the bracts, and the corolla tube is visible, with closed tepals. Harvesting time. |
| Main development stage 6: flowering | |
| 600 | Anthesis of the first basal flower of the first inflorescence. |
| 601 | Medial flowers in the inflorescence are separated from the bracts, the corolla tube is visible, and the tepals are closed. |
| 602 | Anthesis of the basal flowers. |
| 603 | Anthesis of the medial flowers. |
| 604 | Distal flowers of the first inflorescence separated from the bracts, corolla tube visible. |
| 605 | Withering of the basal flowers. |
| 606 | Anthesis of the distal flowers. |
| 607 | Withering of the medial flowers. |
| 609 | End of flowering. All the flowers have withered. |
| 610 | Anthesis of the first basal flower of the second inflorescence. |
| 61. | Stages continue until… |
| 619 | The end of flowering. All the flowers have withered. |
| 621 | Anthesis of the first basal flower of the third inflorescence. |
| 62. | Stages continue until… |
| 629 | The end of flowering. All the flowers have withered. |
| Main development stage 9: Senescence | |
| 901 | Beginning of senescence. Outer leaves are brown. |
| 905 | Progress of the senescence process. Some inner leaves are brown. |
| 909 | All the leaves are going through senescence. The aerial part is dry. |
In the scale suggested in this work, the following stages were omitted: side sprouting/tillering (stage 2), stem elongation (stage 3), fruit development (stage 7), fruit ripening and seed (stage 8). Stages 2 and 3 were not included, as they are not part of freesia growth cycle. Similarly, stages 7 and 8 were not considered, as they are not relevant to the production of cut flowers and corms.
Within each main phase, secondary stages were distinguished, as well as a mesostage between the main stage and the secondary one, so as to describe crop development with a higher precision (Table 1). Since the main stages of sprouting and development of vegetative propagated organ refer to the mother corm and the new corm, respectively, in these cases the mesostage remained the same, and was thus coded as zero (0).
Main development stage 0: sprouting
This stage was described on the basis of observations in the laboratory trials.
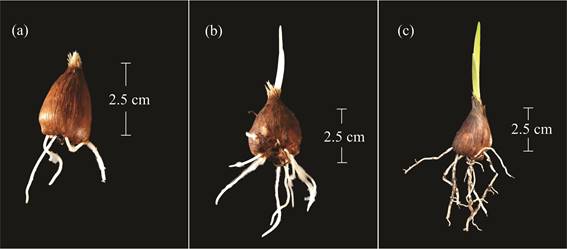
The code 000 on the scale corresponded to the dormancy stage, during which no external morphological changes were observed. Once this stage was over, radicles formed from the base of the corm (code 003) (Table 1), and then fibrous roots emerged, and a conic bud, which was protected by the tunic, became visible on the top of the corm (code 005) (Figure 1a). Subsequently, these roots elongated and the white sprout grew from between the tunics (code 007) (Figure 1b).
This main phase ended when the sprout, which was composed of 2-3 membranous sheaths, emerged from the ground (code 009) (Figure 1c).
Main development stage 1: leaf development
Each new leaf grew out of the apical meristem, specifically developing inside the sprout and emerging from a false stem, which was formed by leaf sheaths. Leaf arrangement was equitant, that is to say, younger leaves were found towards the center of the plant.
Code 101 was used when the leaf blade of the first leaf appeared and measured more than 2 cm long (Figure 2a). Stage 102 was reached when the second leaf blade appeared and was longer than 2 cm, and code 105 was used when the fifth leaf blade appeared and was longer than 2 cm (Figure 2b). Thus, code 109 indicated the appearance of the ninth leaf blade appeared and was longer than 2 cm (Figure 2c); and codes 110 and 112 represented the appearance of the tenth leaf blade and the twelfth leaf blade, respectively, with blades that were longer than 2 cm (Table 1).
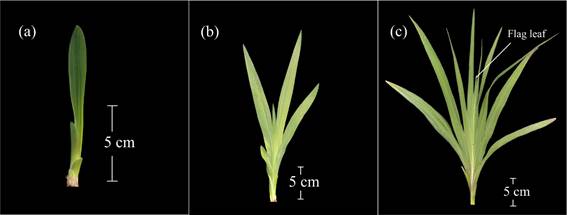
Figure 2 Main development stage 1: leaf development. a: appearance of the first leaf blade (code 101). b: appearance of the fifth leaf blade (code 105). c: plant with nine leaves and with appearance of the flag leaf (code 109/500).
The plants from the first planting date (2/28) produced an average of 11 leaves (code 111), whereas those from the second date (3/21) presented an average of 10 leaves (code 110). The remaining plantations (4/11 and 5/2 planting dates) gave plants with 9 leaves (code 109).
Main development stage 4: development of vegetative propagated organ
This stage was signaled by the appearance of the contractile root at the base of the leaf sheaths, as independent from the root system developed by the planted corm (mother corm) (code 400) (Figure 3a). Later, this root lengthened and the base of the leaf sheaths bulged, which marked the birth of the new corm. The plant remained attached to the mother corm, and continued developing its root system (code 403) (Table 1).
The mother corm started to undergo dehydration, whereas the new corm increased the diameter and the number of roots (code 405) (Figure 3b). When the mother corm was completely dehydrated, and the new corm acquired its maximum size and presented cormels at its base, code 407 was used (Figure 3c). This all happened simultaneously with the senescence of the aerial parts of the plant (Figure 9). Towards the end of this stage corm dormancy began, which implied that this organ was ready for harvest (code 409) (Table 1).
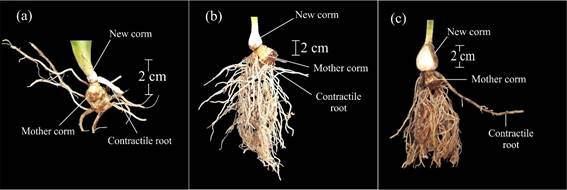
Figure 3 Main development stage 4: development of the vegetative propagated organ. a: development of the contractile root at the base of the sheaths (code 400). b: increase in new corm diameter, beginning of dehydration of the mother corm (code 405). c: complete dehydration of the mother corm. The new corm reaches its final size (code 407).
Main development stage 5: inflorescence emergence
Code 500 was used to indicate that the flag leaf had emerged (and was 2 cm long) (Figure 1c). The first inflorescence was coded with number 501 (Figure 4a), and code 502 represented the lengthening of the flower scape (Table 1). Code 503 was used when it was possible to observe a curvature in the inflorescence at the base of the first flower (Figure 4b). Later, the rachis elongated, and flowers grew more apart from one another (code 504) (Figure 5a). Flower development was acropetal, and the tepals of basal flowers were the first to acquire some color (code 505), followed by the medial tepals (code 506), and finally by the distal ones (code 507) (Figure 5b). This phase finished when the basal flowers split from the bracts that contained them, and the corolla tube became visible, but with closed tepals. This stage corresponded to inflorescence harvesting time (code 509) (Figure 5c).
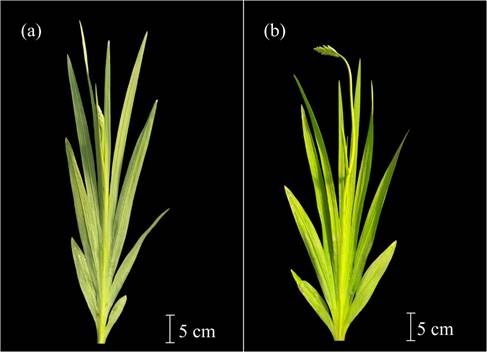
Figure 4 Main development stage 5: inflorescence emergence. a: inflorescence emergence (code 501). b: curvature of inflorescence at the base of the first flower. The base of the inflorescence and the flower scape form a 90-degree angle (code 503).
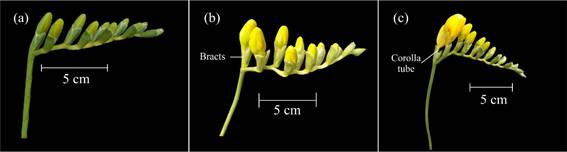
Figure 5 Main development stage 5: inflorescence emergence. a: the rachis has grown, and the flowers in the inflorescence are spaced further apart (code 504). b: distal flowers with pigmented tepals, supported by the bracts and closed (code 507). c: inflorescence ready for harvest. First basal flower of the terminal inflorescence separated from the bracts, with pigmented and closed tepals. The corolla tube is visible (code 509).
Codes 50., 51. and 52. indicated the development of the first, second, and third inflorescences, respectively, which cooccurred (Table 1).
Main development stage 6: flowering
The development, anthesis, and senescence of the flowers in the inflorescences showed an acropetal progression.
Code 600 represented the onset of the phase, with the first basal flower of the inflorescence going through anthesis (Figure 6a). This stage is considered the right time for harvesting flowers, when these are to be sold in markets that are close to production sites.
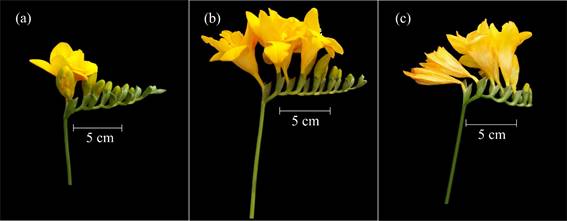
Figure 6 Main development stage 6: flowering. a: anthesis of the first basal flower of the first inflorescence (code 600) b: anthesis of the medial flowers in the first inflorescence (code 603). c: withering of the basal flowers in the first inflorescence (code 605).
Code 601 was used when inflorescence medial flowers separated from the bracts that had kept them inside, with a corolla tube which became visible, and tepals that remained closed. Anthesis of the remaining basal flowers was coded as 602, and that of medial flowers as 603 (Figure 6b).
Code 604 was used when distal flowers grew apart from the bracts they were originally in, exhibiting their corolla tube and closed tepals. The first flowers that withered were the basal ones (code 605) (Figure 6c), and this event took place before the anthesis of the distal flowers (code 606). Subsequently, medial flowers were the ones that withered (code 607), and the end of flowering was reached when distal flowers wilted (code 609) (Table 1).
Codes 60., 61. and 62. were used for coding flowering development in the first, second, and third inflorescences, respectively, all of which took place at the same time (Figure 7).
Main development stage 9: senescence
This stage started when the external leaves, the oldest ones, suffered senescence (code 901). Senescence then progressed, affecting the central leaves next (code 905), and finally all the aerial parts of the plant (code 909). This stage coincided with corm harvesting time (code 409). Dry leave bases formed the tunics that protected the corm during dormancy (Table 1).
The phases of development of the crop did not take place one after the other, as the scale may suggest. On the contrary, some phases even occurred simultaneously (Figure 9). Thus, main development stage 4 stretched throughout the crop cycle and took place simultaneously with leaf development (main development stage 1), inflorescence emergence (main development stage 5), flowering (main development stage 6), and senescence (main development stage 9) (Figure 8).

Figure 8 Simultaneous occurrence of main development stages 1, 4, and 5. The plant reached its vegetative stage with 10 leaves, with the mother corm being totally dehydrated, and the new corm showing its final size. Beginning of reproductive development, with the inflorescence appearing among the leaves (codes 110/407/501).
Discussion
In this work, the scheme of the BBCH scale was adapted to an ornamental crop, the same as was done by Meier et al. (2008) when studying Rosa x hybrida, and by Vela et al. (2013), when analyzing Camellia japonica.
The entire crop cycle of freesia was described and coded, and a phenological scale of 6 main development stages was proposed. This outcome is similar to the phenological description of saffron made by López-Corcoles et al. (2015).
During freesia crop cycle, some stages overlap and occur simultaneously. In agreement with Meier et al. (2009); and Feller et al. (2012), a slant bar (/) was used to indicate the main development stages that took place simultaneously.
The BBCH scale proposed in this work describes its development in a comprehensive way. This resembles what was published by Arena et al. (2013); López-Corcoles et al. (2015); Martínez-Nicolás et al. (2016); López-Bellido et al. (2016); Kishore (2018); Javadzadeh (2018); and Castro et al. (2018), where main development stages were also shown to take place simultaneously. These authors adjusted the BBCH scale to different crops so as to gain a general view of all the development stages.
The proposed scale includes a description of all the morphological changes that both aerial and underground plant organs underwent throughout the crop cycle. Even though Yasmin and Nehvi (2018) based their work on a scale other than the BBCH scale, they also remarked that it was important to describe the development of aerial and underground Crocus sativus organs, since both the corm and the flower are economically important, the same as in the case of the freesia crop. However, despite such importance, Schwab et al. (2015) proposed a phenological scale for Gladiolus grandiflorus which covered the description of morphological changes observed only in the aerial parts of the plant, without considering corm development after the sprouting process.
The methodology used for coding and describing the sprouting stage in the lab was in agreement with the one used Schwab et al. (2015), but differed from the one proposed by López-Corcoles et al. (2015) and López-Bellido et al. (2016), when studying bulbs of other geophytes. These authors collected samples from field trials, and dissected vegetative propagation material to observe the sprouting stage.
The criterion used in this study for establishing that a leaf had appeared, based on leaf blade length, coincided with that proposed by López-Bellido et al. (2016), but differed from the one used by Schwab et al. (2015), who did not set any unit of measure.
Ali et al. (2011) and Al-Shareefi et al. (2019) showed that the number of leaves produced by freesia plants throughout their cycle depends on growth conditions. If plants produce more than 10 leaves during their vegetative stage, the scale proposed in this work covers that situation too, as a mesostage is considered as part of the leaf development stage, occurring between the main and secondary stage. Leaf production was quantified in a similar way in Rosa x hybrida (Meier et al., 2008), Camelia sativa (Martinelli and Galasso, 2011), and Allium sativum crops (López-Bellido et al., 2016).
Freesia flower scape is ramified and its number of inflorescences varies depending on growth conditions (Startek et al., 2004; Wang, 2007; Al-Shareefi et al., 2019). Therefore, in the scale proposed in the present work, a mesostage was introduced in development stages 5 and 6, between the main and secondary stages, in order to describe the development of each one of these inflorescences. Likewise, in the BBCH scale applied to Mangifera indica (Hernández Delgado et al., 2011), the same criterion was considered for describing the development of primary and secondary inflorescences.
Conclusion
The BBCH scale was adapted to describe the phenology of Freesia x hybrida in a comprehensive way, considering both its underground and its aerial organs. The proposed scale identifies 6 main stages.
The description and coding of the development stages of freesia plants constitutes a new contribution and a useful and precise reference tool for identifying the different development stages of the crop, which is essential for commercial production and for making decisions concerning the adoption of crop management techniques to ensure cut flower quality.