Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease in which autoantibodies against self-structures are synthesized1. The etiopathogenesis of this disease is multifactorial, and infections are supposed to play an important role in this context, as microorganisms may stimulate the immune system. Cross-reaction of microorganisms’ structures with self-antigens or molecular mimicry is one of the possible explanations2-4. Moreover, amplified B cell activation results in expanded immune response and increased interferon levels. This cytokine has a crucial role in adequate pathogen response and is important in SLE pathophysiology. Therefore, several infections, mainly viral, have been described just before SLE flares5. Herpes zoster infection6, cytomegalovirus7, and parvovirus B198 are some of them. Recently, the infection by the COVID-19 virus has also been implicated in this setting6,9.
On the other hand, vaccination may offer similar antigens as the original infection, and the risk of SLE reactivation post-vaccination is uncertain10,11. Nevertheless, vaccination is vital for this group of patients as they are immunosuppressed12. Infections are one of the main causes of death in SLE13. Therefore, studies in vaccine safety are of fundamental importance in immune-mediated diseases.
Our objective was to estimate the side effects and the risk of disease flare after COVID-19 vaccination in Brazilian patients with SLE.
Materials and methods
This is a retrospective study performed in a single rheumatology unit that cares for patients from the public health system. A convenience sample included all SLE patients who came for regular consultation from March to September 2021. Patients were invited to participate according to appointment order, which included observing inclusion and exclusion criteria and the patient’s willingness to participate in the study.
To be included, patients should have fulfilled at least 10 points in the 2019 SLE Classification Criteria from ACR/EULAR (American College of Rheumatology/ European League Against Rheumatism). They should also have received two COVID-19 vaccine doses and be older than 18 years of age. Pregnant patients and those unable to understand the consent form were excluded.
We collected epidemiological and clinical data: sex, age, auto-declared ethnic background, comorbidities, and used medication. Data on disease activity in the last consultation prior to the first vaccine dose and in the first consultation after the second vaccine dose were registered using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI). SLEDAI is an instrument that scores 24 clinical and laboratory manifestations; it goes from zero to 105 with high values, which means high disease activity. SLE was considered inactive when SLEDAI is < 414,15. An increase of 3 points in the SLEDAI score was considered as a disease flare16. The study of possible changes in the SLEDAI was done only in patients receiving two doses of the same type of vaccine.
A questionnaire with “Yes/No” answers on the following vaccination side effects was done: local pain, myalgias, fatigue, headache, arthralgias, skin rashes, and nausea/diarrhea. If the answer was “Yes,” the patients were invited to quantify the symptoms as mild, moderate, or severe. Side effects not listed previously were reported as “others” in an open question.
The following questions were developed regarding the patient’s perception of vaccination: a) have you been afraid of receiving the COVID-19 vaccine?; b) according to your perception, do you believe that your disease (SLE) became more active after vaccination?
In statistical analysis, categorical data were expressed in numbers and percentages; numerical data in mean and SD (standard deviation) or median and IQR (interquartile range). Comparison of categorical variables (number of patients with vaccine side effects) was done by Fisher and chi-squared tests, and comparison of numerical variables (SLEDAI) was done through Wilcoxon matched-pairs signed rank test using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com, p<0.05 was considered significant.
This study was approved by the local committee of ethics in research under protocol number 5.123.370 and all participants signed an informed consent.
Results
One hundred and one SLE patients were included. Table 1 shows the epidemiological and clinical data, treatments, and the presence of comorbidities.
Table 1: Characteristics of the lupus patients.
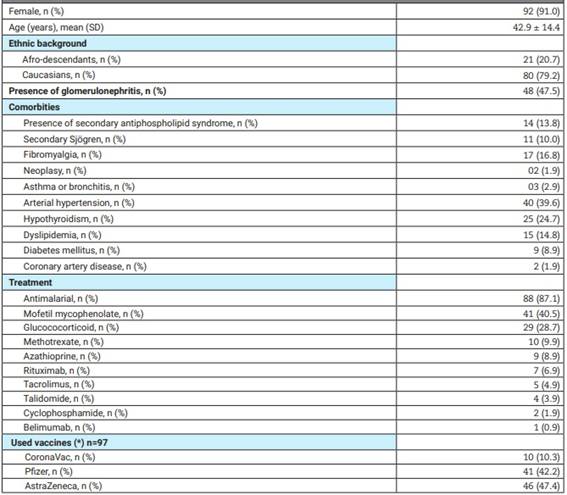
(*) Only those receiving the same vaccine in the first and second dose were considered. N: number, SD: standard deviation.
Side effects post-vaccination were present in 77/101 (76.2%) of the sample, and they are listed in Table 2. Most of them were mild or moderate. Local pain was the most common complaint. None of the interviewed patients had severe side effects, such as severe allergies, anaphylaxis, or thrombosis, and none required hospitalization. The comparison of side effects among the different vaccine types showed no differences (all non-statistically significant).
Table 2: Side effects post COVID-19 vaccination in patients with systemic lupus erythematosus (n=101).
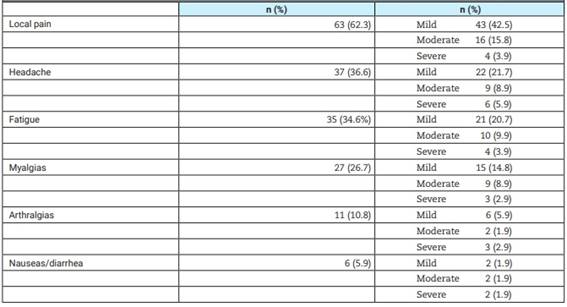
N: number.
About 10/101 (9.9%) patients believed that the SLE became more active after vaccination; 35/101 (34.6%) patients declared being afraid of receiving the first dose of the vaccine. Of those who believed that the SLE became active after the use of the vaccine, only one patient had a change in SLEDAI ≥3.
The median period between the last SLEDAI measurement prior to vaccination and the first vaccine shot was 30 days (IQR=16-51), and the median period between the second shot and the second SLEDAI measurement was 31 days (IQR=30-60).
SLEDAI prior to vaccination ranged from 0-28, with a median value of 0 (IQR=0-2). SLEDAI after vaccination ranged from 0-17, with a median of 0 (IQR=0-0); p=0.68. In 4/97 (4.1%), the SLEDAI increased more than 3 points from baseline, with proteinuria in three patients and arthritis in two. Two of them had active disease when they received the first vaccine dose.
When comparing SLEDAI values prior and after vaccination according to vaccine type, no differences were found for AstraZeneca vaccine (p=0.85), and neither for the CoronaVac (p=0.50). However, a decrease in those receiving the Pfizer vaccine was observed (SLEDAI before vaccination with a median value of 0; IQR=0-2; after vaccination with a median value of 0; IQR=0-0; p=0.04).
Discussion
The present results are reassuring concerning the use of COVID-19 vaccines in SLE patients. No important changes in SLEDAI were observed, and the immediate side effects were comparable to those experienced by the general population. In the current sample, the side effects were similar among the three vaccine types.
Concerns about vaccine safety are common in the rheumatological community. Live vaccines are not indicated in immunocompromised patients such as those with SLE, taking into account the risk of uncontrolled viral replication18. Also, the medical literature has documented the development of de novo autoimmune disease or flares of existing autoimmune disease after vaccine administration18,19. Immunization with the hepatitis B virus (HBV) vaccine in SLE animal models has been shown to accelerate renal disease, and the animals’ kidney histology showed deposition of HBV antigen20. In humans, a case series of SLE developing after they received HBV vaccination has been described, and Agmon-Levin et al observed that they are clinically similar to drug-induced lupus21. Nevertheless, a prospective study in a cohort of 28 SLE patients showed that this vaccine was safe, well-tolerated, and efficacious22.
The human papillomavirus (HPV) vaccine has also been implicated in new-onset lupus. One case-control study using a database from the vaccine adverse event reporting system (VAERS) from 2006 to 2012 reported an OR of 7.6 (95%CI=3.3-19.3) for the development of SLE following the administration of quadrivalent HPV vaccine23. On the other hand, another study involving 113 specialized centers could not prove this association24. As can be seen, controversies about the possibility of lupus appearing after vaccination are common. Regarding COVID-19 vaccination, the toll-like receptor used as an adjuvant has the potential to induce SLE flares, upregulating the type I interferon pathway25. Several autoimmune phenomena have been described following vaccination, such as ANCA associated vasculitis26, myositis27, autoimmune hemolytic anemia28, and thrombocytopenic purpura29, among others. However, the existing data is mostly based on case descriptions. The emergence of autoantibodies after COVID-19 infections has better documentation: anti 52 kDa SSA-Ro/TRIM2130, anti-cardiolipins31, and rheumatoid factors32 are some of them, but the development of autoantibodies after COVID-19 vaccinations is less studied.
COVID-19 vaccination adverse events in SLE patients in the COVAD study33, which included 583 individuals among others with autoimmune diseases, observed minor adverse events in 83% of them, major in 2.6%, and hospitalizations in 0.2% of the sample. This study was done through an internet questionnaire; therefore, details about the mentioned side effects were not available. Moreover, this survey discussed problems occurring immediately (7 days) after vaccination, and the SLEDAI score was not followed17. Another internet survey study aiming to see side effects, including SLE flares, reported that 21/696 (3%) informed having a medically confirmed flare of the disease after a median of 3 days after vaccination33.
Moyon et al34, studying 126 French patients with active and inactive SLE, observed that the BNT162b2 (Pfizer) vaccine was safe, well tolerated, and did not cause any changes in the evaluated activity indexes measured by SLEDAI and BILAG (or British Isles Lupus Assessment Group), corroborating our findings. Furthermore, another report35, including 90 American SLE patients, showed that the pre and postvaccination SLEDAI scores (measured in 55 of them) were similar; flares occurred in 11.4% of patients, and in only one, it was considered severe (with arthritis requiring methotrexate treatment). Mock et al36 compared the number of flares in vaccinated and unvaccinated SLE patients and found no significant differences. SLE is a disease with periods of remitting and recurring activity. Therefore, adjudicating a disease flare to the vaccine is difficult. This cyclic pattern of the disease activity could explain the interesting finding of decreased SLEDAI values observed presently in Pfizer vaccine users.
About one-third of our sample was afraid of receiving the COVID-19 vaccination. COVID-19 vaccines were rushed into testing, bypassing some animal experimentations due to the severity of the infection outbreak37; it is comprehensible that they raised apprehension on efficacy and safety and generated indecision regarding their use. Nevertheless, it must be considered that SLE patients are a high-risk population concerning outcomes associated with SARS-CoV-2 infection not only due to immunosuppression caused by the disease and treatment but also because they have a high number of comorbidities such as hypertension (present in 40% of the present study sample) that is a predictor of hospitalization38. Thus, knowing that COVID-19 vaccination is safe should be reassuring to these populations and to those involved in their care.
This series is limited by the low number of patients and its retrospective design. Moreover, vaccination efficiency was not studied. It is important to mention that Tan et al39 reviewed the data from 32 studies comprising 8269 individuals with SLE showing that post-vaccine COVID-19 severe flares, and adverse events were infrequent. Our cohort has the benefit of showing the local data: that COVID-19 vaccine is safe for local lupus patients in a real-life scenario.
In conclusion, the present results indicate that COVID-19 vaccines are safe and well-tolerable in SLE patients.














