Introduction
Brucellosis continues to be a major public and animal health problem in many regions of the world. This bacterial disease causes reproductive losses resulting from abortion and birth of weak offspring or infertility in all susceptible and accidental hosts.
In most endemic regions, Brucella melitensis is the species most frequently reported as a cause of human disease and the most frequently isolated from cases. However, sporadic cases of human brucellosis due to B. suis have been reported in Brazil, Spain, Germany, India and Polynesia (Guerrier et al., 2011; Meirelles-Bartoli et al., 2012; Naha et al., 2012; Compés Dea et al., 2017; Zange et al. 2019). Brucella suis biovar 1 and B. suis biovar 1a with atypical characteristics have been recognized as the major etiological agent of swine and human brucellosis in South America, mainly in Argentina (Lucero et al., 2008; Escobar et al., 2013). Sixty-eight percent of argentine porcine population is located in the provinces of Buenos Aires, Córdoba and Santa Fe. Likewise, small-scale pig farms (i.e., raising less than 100 sows) represent more than 99% of swine producers (National Service for Animal Health and Food Quality, (SENASA)), operating with low to minimal biosecurity measures with consequent risk for human and animal health, and food or material production. Data on the prevalence of porcine brucellosis in Argentina are scarce. Surveys conducted between 1960 and 1980 found 14.2 to 25% prevalence (Samartino et al., 2002). In 2017, Dibarbora et al. reported that 6% of backyard and small-scale pig farms had seropositive animals. This study also showed that none of the producers was aware of the risk factors concerning the transmission of brucellosis from pigs to humans. Diagnosis of porcine brucellosis is performed mainly by serological tests because bacteriological studies are difficult to apply on animals, since they are costly and due to a dearth of facilities and trained personnel.
Brucellosis proved to be a serious occupational health hazard for veterinarians, veterinary technicians, abattoir workers, farmers and laboratory personnel who are frequently exposed to infected animals, or tissues and fluids from these animals (Corbel, 2006). Other reported routes of transmission are ingestion of inadequately cooked meat, inhalation of aerosols containing B. suis and contact with an open wound. As reported elsewhere, in Argentina, B. suis infections are mainly occupational usually affecting workers of pig slaughterhouses and workers of processing plants (Escobar et al., 2013; Wallach et al., 2016). The symptoms of brucellosis are similar to having the flu, with undulant fever which may progress to a more chronic and debilitating disease. Chronicity and recurring febrile conditions with joint pain are common sequelae. In a series of human cases due to B. suis, clinical presentation and complications, apart from a marked increase in alanine aminotransferase levels, were similar to that observed in patients infected with other Brucella spp, like B. mellitensis and B. abortus (Guerrier et al., 2011). Although is a mandatory notifiable-OIE-listed disease in Argentina, cases of human brucellosis still continue to be underdiagnosed and undernotified (Deodato et al., 2011).
Most infected swine do not demonstrate clinical illness, and abortion is generally a minor component of brucellosis presentation. Also, clinical signs of swine brucellosis include orchitis, lameness and abscess formation in various organs (Olsen et al., 2012). Transmission during copulation is frequent and early abortion with return to oestrus may be the only sign. Animals may also be infected by consumption of feed contaminated with uterine discharges, abortion products or urine from infected animals. After exposure, pigs develop a prolonged bacteremia and B. suis colonizes the reproductive tract. Pig females usually recover promptly after abortion and they can successfully conceive and give birth to live pigs in future gestations. By contrast, infected boars exhibit few signs of infection although they shed the bacterium in urine and their fertility may be reduced. A significant proportion of pigs will recover from the infection, often within 6 months, but many will remain permanently infected (Olsen et al., 2012).
Diagnosis of swine brucellosis is complicated because its incubation time is quite variable and clinical signs may be absent in endemic farms. Although bacteriological isolation remains the gold standard, this would be unpractical under most conditions (Olsen et al., 2012). For this reason control is based on serological testing and segregation, as well as slaughter of infected breeding stock, or the full depopulation of the affected herd (EFSA, 2009). There is not an available vaccine against this disease in most countries. Limitations in sensitivity and specificity of current serological tests restrict its use at a herd level, rather than for individual animals. The Buffered Plate Agglutination Test (BPAT) or Rose Bengal test (RBT) are most reliable in practice for the identification of infected herds, but they should be combined with other tests, such as the Fluorescent Polarization Assay (FPA), to increase its specificity and sensitivity (Di Febo et al., 2012).
In this report, brucellosis due to B. suis was diagnosed in a pig farm, which maintained the infection in an endemic way. This farm was epidemiologically linked to confirmed human brucellosis associated with occupational exposure.
Case description
Case of human brucellosis: Clinical description and diagnosis
A 27-year-old man manifested intermittent and irregular fever (38°C-39°C) with high "spikes" reaching 40°C or even more in the afternoon, profuse night sweating and weakness for which had begun 1 month before. The patient had been working for 6 years in a small-scale pig farm located in Buenos Aires province, Argentina. In an interview, the patient indicated that he had never suffered these symptoms before, and that he had assisted a sow at farrowing 3 weeks before the onset of symptoms (during our study we found this sow positive to all serological tests for brucellosis).
He was attended at the local clinic where laboratory analysis and radiological imaging were prescribed. Number of red blood cell was lower than normal. Ultrasound examination showed enlargement of the spleen (splenomegaly). Positive agglutination reaction in Huddlesson test (titer: 200), BPAT and RBT were obtained with a serum sample. Active brucellosis was diagnosed which was further confirmed through the isolation of B. suis biovar 1 after blood culture at the Brucellosis Reference Laboratory (ANLIS-INEI).
The patient start a course with oral doxycycline (100 mg twice daily) and rifampicin (300 mg twice daily). Unfortunately, we lost contact with him to follow-up the evolution of symptoms.
Description of the small scale-pig farm
The pig farm was located in the periurban area of Balcarce district, Buenos Aires, Argentina and comprised: 22 sows, 3 boars and 25 gilts belonging to Yorkshire-Landrace breed, 25 weaned piglets and 12 hogs. The animals were raised in a semi-extensive and self-replacing system: some of them were confined to a simple pen, while others were maintained free in the field, and received agricultural or waste products for feeding. The production was intended both for subsistence and commercial purpose. Some sows were restrained in farrowed crates during delivery and in the first days of lactation. The animals were not adequately identified and serological testing for infectious diseases, including brucellosis, was not routinely performed. The person who was charged for the activities such as feeding, cleaning pens, checking water, farrowing assistance and slaughtering of piglets for sale did not wear adequate protective clothing (i.e. disposable gloves, gown, rubber boots and eye protection). At the beginning of the study, all animals were identified with ear tags.
Diagnosis of brucellosis in the farm
In order to determine if the source of brucellosis infection was in the small-scale pig farm where the man worked, clinical examination was carried out and records of clinical signs such as abortion, stillbirth, neonatal death, orchitis or lameness were registered. Animals were sampled for serological and bacteriological analysis. All procedures involving animals were approved by the Animal Welfare Committee (act 087/02) of the Facultad de Veterinarias (Universidad Nacional del Centro de la Provincia de Buenos Aires, Tandil, Argentina; http://www.vet.unicen.edu.ar).
Animals did not show any abnormality in external genitalia or joints, neither were records of symptoms compatible with brucellosis in the animals of the farm.
Serological tests
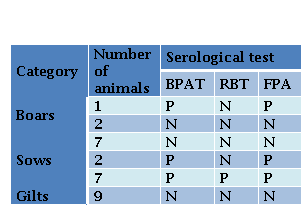
Only animals accessible to capture were sampled. Blood samples from 16 sows, 3 boars and 9 gilts were collected by jugular veinpuncture. Serum samples were analyzed by conventional agglutination tests, BPAT and RBT, and by FPA (Laboratorio Biológico Tandil S.R.L., Argentina). Fluorescence polarization was measured with a FPM-1 Fluorescence Polarization Analyzer and the results were expressed in milipolarization units (mP). Positive or negative results were determined by the presence or absence of visible agglutination (BPA and RBT) and values ≥ 85mP (FPA), respectively. Results were interpreted according to the procedures recommended by SENASA (Nicola et al., 2019).
Specific antibodies were detected in 53 % (10/19) adult pigs, while all sampled gilts were seronegative. Ten animals (9/16 sows and 1/3 boars) were positive to BPAT, and were confirmed by FPA. Seven sows, two boars and all gilts sampled were negative to all tests. In contrast, two sows and the neutered boar, were positive to BPAT and FPA but they were negative to RBT (Table 1).
Bacteriological analysis and Direct Fluorescence Antibody Test (DFAT)
One of the seropositive sows was selected for necropsy, because of its low productivity. Four months after the first serological study, it was bled for the second time and was euthanized using a penetrative captive bolt gun followed by immediate exanguination, and necropsied to perform serological and bacteriological studies. Samples of retropharingeal lymph nodes, tonsils, lung, liver and spleen were obtained and kept in individual sterile plastic bags at -20°C. The seropositive boar was neutered and testicles and epididymis were conserved by the same way. Each organ was homogenized with sterile saline and 1 ml of each sample was seeded onto Brucella Agar and in modified Skirrow´s medium as previously described (Estein et al., 2019). Plates were incubated at 37°C for 10 days in 5% CO. atmosphere. Suspected colonies were identified by Gram staining; catalase, oxidase, urease and nitrate reductase tests and SH. production (Alton et al., 1988).
To detect Brucella by Direct Fluorescent Antibody Test (DFAT), smears from each organ obtained for the bacteriological study were incubated for 1 h with fluorescein-labeled anti-Brucella globulin (Laboratorio Biológico de Tandil S.R.L., Argentina). The smears were visualized by a fluorescence microscope with incident illumination at 100x (Zeiss–Primo Star) (Estein et al., 2019).
Gram negative bacteria were isolated from left testicle and left epididymis of the neutered boar. Isolates were identified as B. suis by phenotypic testing (colony characteristics, catalase and oxidase reactions, nitrate reduction, quick urease reaction and H.S production) and were classified as B. suis biovar 1 by Brucellosis Reference Laboratory (ANLIS-INEI), Argentina. In addition, smears of these tissues were positive to DFAT (Figure 1). All the tissue samples obtained from the necropsied seropositive sow were negative to bacteriological culture, but all tissues gave positive result in DFAT. Paradoxically, while a serum sample from this sow collected 4 months before was positive in all the serological tests, the serum sample obtained at the time of necropsy was negative to both agglutination tests and positive to FPA (Table 2).
Discussion
Brucellosis causes economic losses to the swine industry worldwide (EFSA, 2009). In Argentina, a national register of brucellosis-free establishments was created by SENASA (Resolution 63/2013). However, brucellosis control is not mandatory for small-scale pig producers, which are particularly seated in slums, rural and peri-urban areas (SENASA, 2013). System production of small-holders is usually linked to poor conditions of hygiene and lack of appropriate biosecurity measures, which may promote the contact between the pigs themselves, pigs with other domestic animals (i.e. dogs), and pigs with wild animals. The contact of domestic swine with wildlife reservoirs of B. suis is considered one of the main risks factors associated with porcine brucellosis (Szyfres et al., 1968).
Table 2. Results of serological and direct tests performed on samples from a sow obtained with a time interval of 4 months BPAT Buffer Plate Antigen Test RBT Bengal Rose Test FPA Fluorescence Polarization Assay DFAT Direct Fluorescent Antibody Test P POSITIVE N NEGATIVE
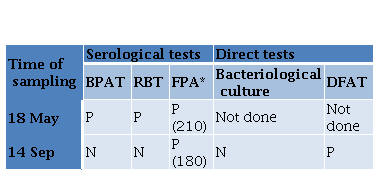
*Millipolarization units given between parentheses.
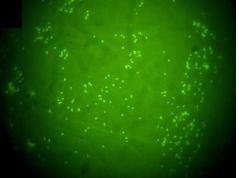
Figure 1. Direct fluorescence antibody test (DFAT) with polyclonal FITC conjugated anti-B. abortus serum. Spleen smear from sow infected with B. suis biovar 1 (100× inmersion oil).
Brucellosis is the world’s most widespread zoonosis (500,000 incident cases of human brucellosis have been reported per year), but also ranks as one of the seven most neglected diseases, according to the World Health Organization (Hull and Schumaker, 2018). Although the true incidence of human brucellosis is unknown in Argentina, of 550 Brucella strains isolated at ANLIS, the reference laboratory for Brucellosis in the country, 11 (20%) were B. canis, 93 (16.9%) were B. abortus, 205 (37.2%) were B. melitensis and 241 (43.8%) B. suis. Of these, 147 (60.9%) were B. suis biovar 1 and 94 (39.0%) were B. suis biovar 1a, which are both endemic in America (Escobar et al., 2013).
In humans, brucellosis caused by B. suis is characterized by non-specific acute symptoms, such as fever, malaise, chills, weight loss and arthralgia. In this study, the patient had the most frequent clinical manifestations and splenomegaly. The time elapsed from initial symptoms to diagnosis was one month coincident with the acute phase of this disease. Brucellosis can evolve to chronic signs, which can affect a large number of systems and cause osteomyelitis, orchitis, hepatitis and endocarditis, among other manifestations (Pappas et al., 2005). In our report, the patient received a classical treatment but we cannot follow-up the evolution of the disease.
The pathophysiology of brucellosis in swine significantly differs from that of brucellosis in large or small ruminants (B. abortus or B. melitensis). Abortion in swine is generally a minor component of the clinical presentation under field conditions (Hutchings et al., 1944, Olsen et al., 2012). The farm described in the present study had not previous clinical or serological records of this disease. However, the serological and bacteriological evidence found in the present study demonstrated that clinically apparently “healthy” pigs carried the infection, in accordance with previous reports (Olsen et al., 2012). Despite agreement in the results of the three serological tests in most of the samples from the swine, 2 sows and 1 boar were positive to BPAT and FPA but negative to RBT. Important differences in the sensitivity/specificity ratios of the serological tests for B. suis have been reported, according to the validation criteria and the different epidemiological conditions. A meta-analysis conducted with data from 5 studies showed that RBT was more sensitive than BPAT when the gold standard was the bacteriological culture (EFSA 2009). In other studies, estimates of sensitivity of RBT were lower compared to FPA (Praud et al., 2013), and also to BPAT (Bence et al., 2018). RBT is considered suitable for the diagnosis of B. abortus infection in cattle, but may not be sensitive enough for B. suis diagnosis in pigs. This may be due, at least in part, to the fact that the agglutination tests and FPA were both mostly developed for detection of the A dominant, B. abortus O side-chain in infected cattle (Palmer and Douglas, 1989; Olsen and Tatum, 2017). Moreover, RBT antigen, unlike BPAT antigen, is standardized without reference to the cell concentration but against an OIESS international reference bovine serum, limiting the sensitivity of the RBT in species different from cattle, like sheep and goats (Blasco et al., 1994).
All gilts were negative in the serological tests. These results were probably associated with reduced susceptibility before sexual maturity and pregnancy, as previously reported in other species (Bekele et al., 2011).
Despite the fact that bacteriological culture is considered to be the gold standard method for the diagnosis of Brucella spp., this method is unfeasible in many situations, because it is time-consuming and hazardous, and the bacterium is difficult to isolate from chronically infected swine (Olsen and Tatum, 2017). We could isolate B. suis biovar 1 from one testicle and epididymis of one boar, but we could not isolate the bacterium from any tissues from the seropositive sow. However, smears of the tissues from both animals were positive to DFAT, confirming that the sow was indeed infected. The difficulty in isolating the bacteria from the sow may be related to sex differences in the recovery of B. suis as reported (Špičić et al., 2013; Olsen and Tatum, 2017).
Noteworthy, at the time of necropsy, specific antibodies in the infected sow could only be detected by FPA, although previous positive serological results were obtained in both the agglutination tests and FPA. We can speculate that, as it has been reported in human and bovine brucellosis, non-agglutinating or blocking antibodies could have been produced associated to a chronic infection, and, therefore, could only be detected by FPA, a primary binding method (Parma et al., 1987).
Although the serological tests performed in this study do not distinguish between smooth Brucella specie. infection, bacteriological results allow us to conclude that B. suis was the etiological agent. B. suis could have been introduced in the farm by the frequent practice of sharing boars for breeding purposes without serological testing and quarantine, which is common in this type of farms.
In view of the results obtained in this study, we advised the owner about control strategies such as quarantine (30 days) for newly purchased animals, serological testing of animals over 45 days at intervals between 30 and 90 days and slaughter of positive animals. Minimal biosecurity practices (i.e. cleaning and disinfection of the pen, feeders and watertroughs with sodium hypochlorite, and wearing protective clothing) were also recommended. In addition, we proposed to delimit clean and dirty zones with perimetral fencing. We also suggested that animals in the clean herd (seronegative) should be fed before those in the dirty herd (seropositive).
From the evidence of endemic infection in the pigs from the farm where the infected human worked and the epidemiological data, we can conclude that the person most likely acquired the disease from the infected animals, or by contact with contaminated environment in the farm. In fact, control strategies such as quarantine, serological screening and slaughter of positive animals, and minimal biosecurity practices had never been applied in the small-scale pig farm where the person worked for 6 years.
The present study highlights the importance of B. suis biovar 1 as a cause of occupational exposure to man in an endemically-infected farm where brucellosis had not been previously detected. In addition, our results underscore the need to improve productor´s education on appropriate biosecurity measures and to actively screen animals for swine brucellosis.