Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cirugía
versión On-line ISSN 2250-639X
Rev. argent. cir. vol.113 no.1 Cap. Fed. abr. 2021
http://dx.doi.org/10.25132/raac.v113.n1.1510.ei
Articles
ALPPS: a historical review
1 Instituto de Investiga ción Sanitaria Aragón, Servicio de Cirugía General y del Aparato Digestivo, Hospital Uni versitario Miguel Servet, Zaragoza, España.
2 Servicio de Cirugía General y del Aparato Digestivo, Hospital Universitario de Guadalajara, Guadalajara, España
Introduction
The current associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure has represented breakthrough in the field of hepato-pancreato-biliary (HPB) surgery1,2.
Surgery remains the only curative option for malignant liver tumors. Large liver resections are commonly required to achieve clear surgical margins. Thus, an adequate volume and future liver remnant (FLR) is mandatory, as posthepatectomy liver failure (PHLF) is one the main causes of morbidity and mortality after extensive liver resections3,4. Classically, at least two anatomically contiguous liver segments had to be preserved, representing a critical minimum FLR of 20-25%4,5, and of 40% in the presence of chronic liver disease. ALPPS broke this paradigm4,5.
Material and methods
We conducted a bibliographic search in PubMed using the term “ALPPS”, and a clustering approach selecting the articles by their relevance and historical influence on the technique.
Techniques for liver regeneration before ALPPS
Over the past decades, two methods have been developed to induce hypertrophy of the FLR: portal vein occlusion (PVO) through embolization (PVE) or ligation (PVL) and two-stage hepatectomy (TSH)6; both methods are usually used in association. PVE is currently the gold standard method. In the eighties, Makuuchi7 introduced this new concept: right PVE to induce hypertrophy of the left liver lobe to safely resect large or multiple tumors. However, hypertrophy of the FLR is not always sufficient resulting in PHLF. Even more, the disease may accelerate in some patients after PVE4, 8. Up to 40% of the patients are not candidates for surgery for these reasons4,9.
Two-stage hepatectomy was described by Adam et al.10 in 2000 to treat patients with bilobar involvement due colorectal liver metastases (CRLM) initially unresectable. During first-stage hepatectomy the greatest number of metastases are resected, and after a period of hypertrophy of the FLR and systemic chemotherapy, the second hepatectomy can be carried out as a curative intent procedure. Morbidity is between 49% and 59% and mortality ranges between 6.4% and 8.8%4. Complete resection is not achieved in about 25% of the patients11.
First procedures of portal vein ligation and liver partition
In 2007, Hans Schlitt performed the technique that was later known as ALPPS for the first time in a patient with Bismuth type IV hilar cholangiocarcinoma. The first report was a case series of three patients presented by Lang at the 9th congress of the E-AHPBA (European-African Hepato-Pancreato-Biliary Association) in Cape Town, South Africa, in 2011, which generated significant excitement and controversy12.
In 2011, Alvarez et al.13 published a new technique for liver regeneration performed on a patient with colorectal cancer and initially unresectable liver metastases: left hemicolectomy, cleaning of the metastases in the left liver and in situ liver split with right PVL. The procedure also included ligation of the right bile duct, a gesture that was later associated with increased risk of bile leak14. They described a 40% increase of the FLR on postoperative day 6 and completed the right liver resection on day 7; the patient was discharged 11 days later.
In 2012, Schnitzbauer et al.5 published a retrospective review of 25 cases, mostly of CRLM, treated between 2007 and 2011 with a 2-step technique. In the first step, right portal vein ligation and in situ splitting of the liver parenchyma along the falciform ligament were performed. The right lobe was covered in a plastic bag to prevent adhesions. After a median interval of 9 days (range: 5-28 days), hypertrophy of about 75% of the left liver lobe was induced and volumetry was performed. In the second step, patients underwent right trisectorectomy (Fig. 1).
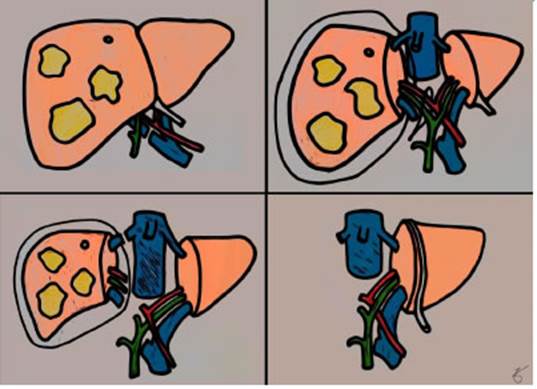
Figure 1 Initial ALPPS technique. Top, left (first stage): liver tumors mainly in the right liver. Right portal vein ligation. Top, right (first stage): in situ split of the right liver in plastic bag; a suture is placed to tag the right hilum. Bottom, left (second stage): li gation of the right hepatic hilum and right hepatic vien. Bottom, right (second stage): fi nal result, with hypertrophy of the FLR.
Sixty-four percent of the patients presented complications. Of the 50 complications documented, 22 (44%) were classified as grade III or IV of the Clavien- Dindo classification. Mortality rate was 12%.
The technique was initially described as “2-staged extended right hepatectomy with initial surgical exploration, right PVL, and in situ splitting”. In 2012, de Santibañes and Clavien5 proposed the acronym “ALPPS” to describe this novel approach (Table 1).
Expansion and international registry
The approach aroused considerable interest16, and an international registry created in 2012 recruited more than 1200 cases17. The registry contributed to monitoring the results, improving patient selection and analyzing complications and their possible solutions.
The first report published data of 202 patients retrieved from the registry until January 201418. Overall survival (OS) at 1 and 2 years was 73% and 59%, respectively, and disease-free survival (DFS) was 60% and 42%, respectively, with mean survival rate of 24 months. Mortality at 90 days was 9%. Predictors for severe complications were primary liver tumors, age > 60 years, need for intraoperative red blood cell transfusions and operative time > 5 hours. Patients with cholangiocarcinoma and gallbladder cancer had worse outcomes18, probably due to the high rate of biliary complications4.
The registry was also useful to analyze the indications of ALPPS19. Records from 2012 to 2017 were screened, considering that patients with a remnant liver to body weight ratio ≥ 0.5% in healthy livers and ≥ 0.8% in case of liver damage could undergo one-stage hepatectomy, and thus, a right liver resection could have avoided such additional risk. The authors concluded that about one third of patients undergoing ALPPS did not fulfill the criteria. This article has reopened the debate that emerged from the beginning, since many authors consider that ALPPS has almost become a fashion that has increased its misuse and should be understood as another option available for surgeons20.
Modifications of the original technique
Many technical variations of ALPPS have been described since the initial description:
▪▪Minimally invasive surgery (MIS) for ALPPS. In 2012, Machado et al.21 performed the first laparoscopic ALPPS, and Vivent at al.22 published the first robotic ALPPS in 2016 The systematic review by Melandro et al.23 included 27 patients undergoing MIS-ALPPS using a laparoscopic or robotic approach; 15.4% of the patients presented grade III complications, and no deaths were reported. In their review, Michal et al.24 compared 46 patients undergoing MIS-ALPPS versus 1088 treated with open surgery. Operative parameters (time and blood loss) did not differ between the two groups. Open ALPPS patients had a more diverse profile of underlying comorbidities, more right extended hepatectomies, and higher rate of severe complications and mortality. In conclusion, there is still limited evidence.
▪▪Tourniquet-ALPPS (ALTPS). The procedure, described by Roblesetal.25 in 2012, consists of right portal vein ligation and applying a tourniquet on the transection line, accelerating hypertrophy, and achieving efficient regeneration with acceptable morbidity and mortality rates26.
▪▪Modified ALTPS27. The technique was designed for perihilar tumors requiring a “non-touch” technique to avoid left portal ligation; right PVE is performed on postoperative day 4.
▪▪Monosegment ALPPS. In 2015, Montalvá Oron et al.28 and Schadde et al.6 published a case series of monosegment ALPPS in 8 and 12 patients, respectively. In the first case series, morbidity was 50%, mortality 25% and disease recurrence was 100%, with median DFS of 155 days 28. In the second series, there were no deaths and 33.3% of the patients presented grade III complications, while disease recurrence was 50% after 14-month follow-up6.
▪▪RALPP (radiofrequency-assisted ALPPS). The approach published by Gall et al.29 in 2015 is through laparoscopic ALPPS with the use of radiofrequency ablation (RFA) to create a line of necrosis through the future transection line. This technique stops blood flow from the FLR to the contralateral liver while inducing hypertrophy of the FLR without a physical partition of the parenchyma.
▪▪LAPS (laparoscopic microwave ablation and portal vein ligation for staged hepatectomy). Gringeri et al.30 described this technique in 2015, which is similar to RFA but uses microwave ablation for liver partition.
▪▪Mini-ALPPS. In 2016, de Santibañes et al.1 suggested that partial parenchymal transection combined with intraoperative portal vein embolization (up to the middle hepatic vein) minimized the impact of the first stage and improved recovery compared with complete liver partition (up to the inferior vena cava). There were no serious complications and an adequate FLR was achieved4.
▪▪PALPP (percutaneous microwave ablation liver partition and portal vein embolization). De Hong et al.31 published the use of percutaneous liver partition using microwave ablation in 2016.
▪▪Hybrid-ALPPS32: Li. et al.32 described this technique in 2016 for tumors with infiltration to the right portal vein in which portal vein ligation is not feasible. They performed a “non-touch” technique with in-situ split using anterior approach, right PVE through interventional radiology (even through the ileocolic vein) without touching the hepatoduodenal ligament and the right liver, and finally completing 2-stage hepatectomy with transection of the portal bifurcation.
▪▪TIPE_ALPPS (transileocecal portal embolization): Sakamoto et al.33 documented this technique in 2016. Portal vein embolization is performed via the ileocecal vein avoiding the hepatoduodenal ligament with partial partition of the liver along the Rex-Cantlie line.
▪▪PRALPPS (percutaneous radiofrequency-assisted liver partition): this technique was described in a case-control study by Melekhina et al.34 in 2020 in patients with perihilar cholangiocarcinoma. The first stage of PRALPPS included percutaneous embolization of the right portal vein guided by ultrasonography and RFA along the right side of the middle hepatic vein under general anesthesia. Thermal ablation involved around 50% of the future transection plane, retaining a minimum distance of 2 cm from the hilar plate to avoid injury.
Because many variants of the ALPPS procedure emerged, Linecker et al.2 proposed the development of a consensus terminology: Rescue-ALPPS (ALPPS after failure of PVE to induce hypertrophy), laparoscopic ALPPS (use of laparoscopy in any stage), PVE-ALPPS (use of PVE as part of the first stage), partial ALPPS (used for incomplete transection: ALTPS, RALPP and LAPS) or monosegment ALPPS.
Expert meetings
First International Expert Meeting
The first expert meeting on ALPPS was held in Hamburg, Germany, in 201516. The meeting produced eight recommendations:
▪▪Knowledge of individual hepatic anatomy is crucial. All efforts should be made to preserve arterial inflow and venous drainage of the “deportalized” part of the liver. Every unnecessary manipulation of the hilum should be strictly avoided.
▪▪No technical variation of the transection technique can currently be recommended as superior.
▪▪A cholangiogram can simplify visualizing biliary tree anatomy and possible bile leaks. Tagging anatomical structures can be performed to facilitate stage 2.
▪▪The first liver volumetry should be done on days 8 to 10 and repeated weekly for 4 weeks if FLR is insufficient.
▪▪CRLM is the main indication. Caution is called to use ALPPS for hepatocellular carcinoma (HCC) and cholangiocarcinoma, in view of the associated higher morbidity and mortality.
▪▪ALPPS is a viable option in cases with very small FLR or bilobar lesions, as drop-out rates seem to be lower compared with PVE or TSH. A ‘‘rescue’’ ALPPS procedure can be feasible in these cases.
▪▪With MELD score >10, step 2 should be postponed. Age > 60 years is associated with higher risk of poor outcomes and may justify longer intervals between both stages.
▪▪ALPPS in CRLM should only be performed after neoadjuvant systemic therapy. Tumor progression on chemotherapy is a contraindication for ALPPS. In case of synchronous colorectal liver tumors, the liver-first approach can be a good option. Caution is given to balance performing simultaneous procedures in liver and bowel.
The “10th anniversary of ALPPS” expert meeting
The 10th anniversary of ALPPS was celebrated during the 12th congress of the E-AHPBA (Mainz, Germany, 2017) at an expert meeting to advance in establishing clear indications, selection of patients and standardization of the surgical technique35. The main conclusions of the meeting are:
▪▪New technical modifications, minimizing the extensiveness of the first-stage procedure, are associated with significant improvements in safety.
▪▪The degree of FLR hypertrophy in fibrotic/cirrhotic livers appears somewhat less substantial than in noncirrhotic livers.
▪▪Resection rates are higher with ALPPS compared with TSH, with similar perioperative morbidity and mortality.
▪▪ALPPS is effective after failure of portal vein embolization.
Current situation of ALPPS
This procedure has demonstrated to offer an opportunity for cure to patients who, initially, have no surgical option11,15,23,36. In the meta-analysis by Moris et al.11, complete resection was achieved in 90.9% of the patients undergoing ALPPS compared with 74.6% in those undergoing TSH. Some studies did not find differences in OS and DFS between both techniques11,37,38. However, the results of the LIGRO trial39,49 demonstrated that ALPPS improved survival in patients with CRLM compared with TSH, with higher resection and survival rates.
The rate of complications and mortality associated with the technique is a matter of concern. Linecher at al.41 validated two risk models for predicting 90-day mortality, and Raptis et al.42 established the benchmark values for ALPPS.
There are no data demonstrating that liver volume increase runs parallel to liver function43. Most deaths due to PHLF occur after the second stage. Thus, a dilemma emerges about the optimal timing for the second procedure. The meeting in Hamburg determined that minimum values for proceeding to stage 2 were FLR > 30% or a FLR/body weight ratio (FLR/BW) > 0.5%, and FLR > 40% or FLR/BW > 0.8%, depending on parenchymal quality4,16. The first CT scan should be done on days 8 to 10 after stage 1 and repeated weekly for 4 weeks, if FLR is insufficient44. Functional tests such as scintigraphy or indocyanine green test should become routine before completing the second stage. They can be especially useful in patients with a borderline FLR to decide the best timing for the intervention and to prevent PHLF. Serenari et al.45 performed hepatobiliary iminodiacetic acid (HIDA) scintigraphy combined with single photon emission-computed tomography (SPECT) before ALPPS stage 2 and developed the HIBA index (HIBA-i), which represents the proportion of radionuclide accumulated in the FLR. None of the patients with a cut-off value of 15% or greater developed PHLF.
The results obtained differ according to the disease that led to surgery:
CRLM
Colorectal liver metastasis is the main indication for ALPPS18,39,46-53 and has been described as independent predictor of lower rate of serious complications (Clavien-Dindo IIIa or greater) > 36%. Overall survival at 1 and 2 years is 76% and 62%, respectively, with DFS of 59% and 41%, respectively18. The LIGRO trial39 is a multicenter, randomized control trial (RCT) that included 97 patients with CRLM randomized in a 1:1 manner to ALPPS or TSH. The resection rate for patients randomized to ALPPS was 92% versus 57% in those undergoing TSH (p < 0.0001). ALPPS induced greater hypertrophy of the FLR (ALPPS: 92% vs. TSH: 57%, p < 0.0001). There were no significant differences in the rate of serious complications (≥ IIIa) (ALPPS: 43% vs. TSH: 43%, p = 1) or 90-day mortality (ALPPS: 9,1% vs. TSH: 10.7%, p = 0.64). Time between stage 1 and stage 2 was longer in TSH (ALPPS: 11 ± 11 vs. TSH: 43 ± 15 days, p < 0.0001). Hasselgren et al.40 described median survival of 46 months for ALPPS vs. 26 months for TSH (p = 0.028).
HCC
Hypertrophy of the FLR develops less rapid and to a lesser extent (40-47% vs. 76-138%) in livers with HCC54-56 according to the degree of fibrosis. Mortality ranges between 9%56 and 31%54, so ALPPS is recommended in HCC with low degree of fibrosis and with a longer interval between stage 1 and 2 to allow the development of sufficient hypertrophy9. In these patients, partial split does not seem to induce sufficient hypertrophy compared with complete split (17.5 mL/day vs. 31.2 mL/day, p = 0.022)56. ALPPS presents better OS and DFS than transcatheter arterial chemoembolization (TACE)57 and higher resection rate than PVE58.
Cholangiocarcinoma
Patients operated on due to cholangiocarcinoma present high morbidity and mortality. The OS is higher with ALPPS compared with palliative chemotherapy59 at 1, 2 and 3 years: 82.4%, 70.5% and 39.6%, respectively versus 51.2%, 21.4% and 11.3% (p < 0.01). This benefit is not observed in those patients with PHLF or multifocal intrahepatic cholangiocarcinoma. The rate of serious complications is 6.1% after stage 1, 41.1% after stage 2, and mortality is 21.2%.
The initial results reported for perihilar cholangiocarcinoma described mortality rate between 40%60 and 48% at 90 days and OS of 6 months61,62. The use of TIPE-ALPPS63 and PRALPPS34 have been reported for these tumors. Olthof et al.62 reported the results of cases of perihilar cholangiocarcinoma included in the ALPPS registry; 90-day mortality was 48% and mean OS was 6 months. FLR hypertrophy induced by ALPPS was similar to the one produced by major hepatectomies, but mortality was higher, and OS was lower. Thus, the authors do not recommend ALPPS in perihilar cholangiocarcinoma and suggest PVE to increase the FLR.
ALPPS in pediatrics
The first indication of ALPPS in pediatrics was reported by Chan et al.64 in 2014. In 2015, Wiederkehr et al.65 published a series of 5 patients without serious complications or deaths.
Conclusions
The ALPPS procedure has generated considerable interest despite its significant morbidity and mortality. A better selection of patients has been possible, along with the definition of timing for the second stage and improved surgical techniques, which have led to acceptable rate of complications and mortality compared with standard liver resections. The technical modifications, have minimized the extensiveness of the first-stage procedure and are associated with significant improvements in safety, preserving a high rate of curative resection. The second stage must be delayed or even not performed in case of clinical impairment, complications or insufficient FLR. Recent data have confirmed higher resection rates achieved with ALPPS than with the standard procedures. ALPPS is an already established concept; nevertheless, several questions remain unanswered, particularly in terms of long-term oncological outcomes. ALPPS does not replace other technique but expands the therapeutic armament.
Referencias bibliográficas /References
1. de Santibañes E, Álvarez FA, Ardiles V, Pekolj J, de Santibañes M. Inverting the ALPPS paradigm by minimizing first stage im pact: the Mini-ALPPS technique. Langenbeck’s Arch Surg. 2016; 401:557-63. doi:10.1007/s00423-016-1424-1. [ Links ]
2. Linecker M, Kron P, Lang H, de Santibañes E, Clavien P-A. Too Many Languages in the ALPPS: Preventing Another Tower of Babel? Ann Surg. 2016; 263:837-8. doi:10.1097/SLA.0000000000001632. [ Links ]
3. Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systema tic liver volumetry. Ann Surg . 2009; 250:540-8. doi:10.1097/SLA.0b013e3181b674df. [ Links ]
4. de Santibañes M, Boccalatte L, de Santibañes E. A literature re view of associating liver partition and portal vein ligation for sta ged hepatectomy (ALPPS): so far, so good. Updates Surg. 2017; 69:9-19. doi:10.1007/s13304-016-0401-0. [ Links ]
5. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg . 2012; 255:405-14. doi:10.1097/SLA.0b013e31824856f5. [ Links ]
6. Schadde E, Malagó M, Hernández-Alejandro R, Li J, Abdalla E, Ardiles V, et al. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015; 157:676-89. doi:10.1016/j.surg.2014.11.015. [ Links ]
7. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990; 107:521-7. [ Links ]
8. Hayashi S, Baba Y, Ueno K, Nakajo M, Kubo F, Ueno S, et al. Acce leration of primary liver tumor growth rate in embolized hepatic lobe after portal vein embolization. Acta Radiol. 2007; 48:721-7. doi:10.1080/02841850701424514. [ Links ]
9. Au KP, Chan ACY. Current status of associating liver partition with portal vein ligation for staged hepatectomy: Comparison with two-stage hepatectomy and strategies for better outcomes. World J Gastroenterol. 2019; 25:6373-85. doi:10.3748/wjg.v25.i43.6373. [ Links ]
10. Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-sta ge hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg . 2000; 232:777-85. doi:10.1097/00000658-200012000-00006. [ Links ]
11. Moris D, Ronnekleiv-Kelly S, Kostakis ID, Tsilimigras DI, Beal EW, Papalampros A, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Sys tematic Review and Meta-Anal. World J Surg. 2018; 42:806-15. doi:10.1007/s00268-017-4181-6. [ Links ]
12. Abstracts of the 9th Congress of the European-African Hepato- Pancreato-Biliary Association (E-AHPBA). April 12-16, 2011. Cape Town, South Africa. HPB (Oxford). 2011;13 (Suppl 2):1-145. doi:10.1111/j.1477-2574.2011.00308.x. [ Links ]
13. Álvarez FA, Iniesta J, Lastiri J, Ulla M, Bonadeo Lassalle F, de Santi bañes E. Nuevo método de regeneración hepática. Cir Esp.. 2011; 89:645-9. doi:10.1016/j.ciresp.2011.08.001. [ Links ]
14. Edmondson MJ, Sodergren MH, Pucher PH, Darzi A, Li J, Petrows ky H, et al. Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): Many routes to the summit. Surgery. 2016; 159:1058-72. doi:10.1016/j. surg.2015.11.013. [ Links ]
15. de Santibañes E, Clavien P-A. Playing Play-Doh to prevent posto perative liver failure: the “ALPPS” approach. Ann Surg . 2012; 255:415-7. doi:10.1097/SLA.0b013e318248577d. [ Links ]
16. Oldhafer KJ, Stavrou GA, van Gulik TM, Core Group. ALPPS--Where Do We Stand, Where Do We Go?: Eight Recommendations From the First International Expert Meeting. Ann Surg . 2016; 263:839- 41. doi:10.1097/SLA.0000000000001633. [ Links ]
17. (ALPPS) W registry of associating liver partition and portal vein ligation for staged hepatectomy. Worldwide registry of associa ting liver partition and portal vein ligation for staged hepatectomy (ALPPS) n.d. www.alpps.net. [ Links ]
18. Schadde E, Ardiles V, Robles-Campos R, Malago M, Macha do M, Hernández-Alejandro R, et al. Early survival and safe ty of ALPPS: first report of the International ALPPS Registry. Ann Surg . 2014; 260:829-36; discussion 836-8. doi:10.1097/SLA.0000000000000947. [ Links ]
19. Schnitzbauer AA, Schadde E, Linecker M, Machado MA, Adam R, Malago M, et al. Indicating ALPPS for Colorectal Liver Metastases: A Critical Analysis of Patients in the International ALPPS Registry. Surgery. 2018; 164:387-94. doi:10.1016/j.surg.2018.02.026. [ Links ]
20. Schlitt HJ, Hackl C, Lang SA. “In-Situ Split” Liver Resection/ALPPS - Historical Development and Current Practice. Visc Med. 2017; 33:408-12. doi:10.1159/000479850. [ Links ]
21. Machado MAC, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg . 2012; 256:e13; author reply e16-9. doi:10.1097/SLA.0b013e318265ff2e. [ Links ]
22. Vicente E, Quijano Y, Ielpo B, Fabra I. First ALPPS procedure using a total robotic approach. Surg Oncol. 2016; 25:457. doi:10.1016/j.suronc.2015.10.001. [ Links ]
23. Melandro F, Giovanardi F, Hassan R, Larghi Laureiro Z, Ferri F, Rossi M, et al. Minimally Invasive Approach in the Setting of ALPPS Pro cedure: a Systematic Review of the Literature. J Gastrointest Surg. 2019; 23:1917-24. doi:10.1007/s11605-018-04092-x. [ Links ]
24. Michal K, Sau M, Tamara GMH, Long JR. A better route to ALPPS: minimally invasive vs open ALPPS. Surg Endosc. 2020; 34:2379-89. doi:10.1007/s00464-020-07437-3. [ Links ]
25. Robles Campos R, Parrilla Paricio P, López Conesa A, Marín Hernández C, García Pérez R, Fuster Quiñonero M. Una nueva estrategia quirúrgica para metástasis hepáticas bilobares múl tiples: oclusión portal derecha y torniquete en la línea de sec ción parenquimatosa. Cir. Esp. 2012; 90:191-6. doi:10.1016/j.ciresp.2012.01.002. [ Links ]
26. Robles R, Parrilla P, López-Conesa A, Brusadin R, de la Peña J, Fuster M, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedu re. Br J Surg. 2014; 101:1129-34; discussion 1134. doi:10.1002/ bjs.9547. [ Links ]
27. Robles Campos R, Brusadin R, López Conesa A, Parrilla Paricio P. Staged liver resection for perihilar liver tumors using a tourniquet in the umbilical fissure and sequential portal vein embolization on the fourth postoperative day (a modified ALTPS). Cir Esp . 2014; 92:6826.doi: 10.1016/j.ciresp.2014.07.006. [ Links ]
28. Montalvá Orón EM, Maupoey Ibáñez J, Bañuelos Carrillo R, Boscà Robledo A, Orbis Castellanos JF, Moya Herraiz Á, et al. Monoseg ment ALPPS: A new variant of the techniques for rapid hepatic regeneration. Critical review of the initial results of our series. Cir Esp . 2015; 93:436-43. doi:10.1016/j.ciresp.2015.02.011. [ Links ]
29. Gall TMH, Sodergren MH, Frampton AE, Fan R, Spalding DR, Habib NA, et al. Radio-frequency-assisted Liver Partition with Portal vein ligation (RALPP) for liver regeneration. Ann Surg . 2015; 261:e45-6. doi:10.1097/SLA.0000000000000607. [ Links ]
30. Gringeri E, Boetto R, DʼAmico FE, Bassi D, Cillo U. Laparoscopic mi crowave ablation and portal vein ligation for staged hepatectomy (LAPS): a minimally invasive first-step approach. Ann Surg . 2015; 261:e42-3. doi:10.1097/SLA.0000000000000606. [ Links ]
31. Hong DF, Zhang YB, Peng SY, Huang DS. Percutaneous Microwave Ablation Liver Partition and Portal Vein Embolization for Rapid Li ver Regeneration: A Minimally Invasive First Step of ALPPS for He patocellular Carcinoma. Ann Surg . 2016; 264:e1-2. doi:10.1097/SLA.0000000000001707. [ Links ]
32. Li J, Kantas A, Ittrich H, Koops A, Achilles EG, Fischer L, et al. Avoid “All-Touch” by Hybrid ALPPS to Achieve Oncological Efficacy. Ann Surg . 2016; 263:e6-7. doi:10.1097/SLA.0000000000000845. [ Links ]
33. Sakamoto Y, Inagaki F, Omichi K, Ohkura N, Hasegawa K, Kokudo N. Associating Liver Partial Partition and Transileocecal Portal Vein Embolization for Staged Hepatectomy. Ann Surg . 2016; 264:e21-2. doi:10.1097/SLA.0000000000001757. [ Links ]
34. Melekhina O, Efanov M, Alikhanov R, Tsvirkun V, Kulezneva Y, Ka zakov I, et al. Percutaneous radiofrequency-assisted liver partition versus portal vein embolization before hepatectomy for perihi lar cholangiocarcinoma. BJS Open. 2020; 4:101-8. doi:10.1002/bjs5.50225. [ Links ]
35. Lang H, de Santibañes E, Schlitt HJ, Malagó M, van Gulik T, Machado MA, et al. 10th Anniversary of ALPPS-Lessons Lear ned and quo Vadis. Ann Surg . 2019; 269:114-9. doi:10.1097/ SLA.0000000000002797. [ Links ]
36. Schadde E, Schnitzbauer AA, Tschuor C, Raptis DA, Bechstein WO, Clavien P-A. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver par tition and portal vein ligation for staged hepatectomy. Ann Surg Oncol. 2015; 22:3109-20. doi:10.1245/s10434-014-4213-5. [ Links ]
37. Ratti F, Schadde E, Masetti M, Massani M, Zanello M, Serenari M, et al. Strategies to Increase the Resectability of Patients with Co lorectal Liver Metastases: A Multi-center Case-Match Analysis of ALPPS and Conventional Two-Stage Hepatectomy. Ann Surg On col. 2015; 22:1933-42. doi:10.1245/s10434-014-4291-4. [ Links ]
38. Kambakamba P, Linecker M, Álvarez FA, Samaras P, Reiner CS, Rap tis DA, et al. Short Chemotherapy-Free Interval Improves Oncolo gical Outcome in Patients Undergoing Two-Stage Hepatectomy for Colorectal Liver Metastases. Ann Surg Oncol. 2016; 23:3915-23. doi:10.1245/s10434-016-5419-5. [ Links ]
39. Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, et al. ALPPS Improves Resectability Compared with Conventio nal Two-stage Hepatectomy in Patients with Advanced Colorectal Liver Metastasis: Results from a Scandinavian Multicenter Rando mized Controlled Trial (LIGRO Trial). Ann Surg . 2018; 267:833-40. doi:10.1097/SLA.0000000000002511. [ Links ]
40. Hasselgren K, Røsok BI, Larsen PN, Sparrelid E, Lindell G, Schultz NA, et al. ALPPS Improves Survival Compared With TSH in Pa tients Affected of CRLM: Survival Analysis From the Randomized Controlled Trial LIGRO. Ann Surg . 2019;Publish Ah. doi: 10.1097/SLA.0000000000003701. [ Links ]
41. Linecker M, Kuemmerli C, Kambakamba P, Schlegel A, Muiesan P, Capobianco I, et al. Performance validation of the ALPPS risk mo del. HPB. 2019; 21:711-21. doi:10.1016/j.hpb.2018.10.003. [ Links ]
42. Raptis DA, Linecker M, Kambakamba P, Tschuor C, Müller PC, Had jittofi C, et al. Defining Benchmark Outcomes for ALPPS. Ann Surg . 2019; 270:835-41. doi:10.1097/SLA.0000000000003539. [ Links ]
43. Olthof PB, Schnitzbauer AA, Schadde E. The HPB controversy of the decade: 2007-2017 - Ten years of ALPPS. Eur J Surg Oncol . 2018; 44:1624-7. doi:10.1016/j.ejso.2018.06.005. [ Links ]
44. Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Le surtel M, et al. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Regis try. Ann Surg . 2015; 262:780-5; discussion 785-6. doi:10.1097/SLA.0000000000001450. [ Links ]
45. Serenari M, Collaud C, Álvarez FA, de Santibañes M, Giunta D, Pe kolj J, et al. Interstage Assessment of Remnant Liver Function in ALPPS Using Hepatobiliary Scintigraphy: Prediction of Posthepa tectomy Liver Failure and Introduction of the HIBA Index. Ann Surg . 2018; 267:1141-7. doi:10.1097/SLA.0000000000002150. [ Links ]
46. Álvarez FA, Ardiles V, De Santibañes M, Pekolj J, De Santibañes E. Associating liver partition and portal vein ligation for staged hepa tectomy offers high oncological feasibility with adequate patient safety: A prospective study at a single center. Ann Surg . 2015; 261:723-32. doi:10.1097/SLA.0000000000001046. [ Links ]
47. Adam R, Imai K, Castro Benítez C, Allard M-A, Vibert E, Sa Cunha A, et al. Outcome after associating liver partition and portal vein liga tion for staged hepatectomy and conventional two-stage hepatec tomy for colorectal liver metastases. Br J Surg . 2016; 103:1521-9. doi:10.1002/bjs.10256. [ Links ]
48. Tanaka K, Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur JSurg Oncol . 2015; 41:506-12. doi:10.1016/j.ejso.2015.01.031. [ Links ]
49. Croome KP, Hernández‐Alejandro R, Parker M, Heimbach J, Rosen C, Nagorney DM. Is the liver kinetic growth rate in ALPPS unpre cedented when compared with PVE and living donor liver trans plant? A multicentre analysis. HPB . 2015; 17:477-84. doi:10.1111/ hpb.12386. [ Links ]
50. Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Ya mazaki K, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 2016; 159:1289-98. doi:10.1016/j.surg.2015.12.004. [ Links ]
51. Chia DKA, Yeo Z, Loh SEK, Iyer SG, Madhavan K, Kow AWC. ALPPS for Hepatocellular Carcinoma Is Associated with Decreased Li ver Remnant Growth. J Gastrointest Surg . 2018; 22:973-80. doi:10.1007/s11605-018-3697-x. [ Links ]
52. Shindoh J, Vauthey J-N, Zimmitti G, Curley SA, Huang SY, Mahvash A, et al. Analysis of the Efficacy of Portal Vein Embolization for Pa tients with Extensive Liver Malignancy and Very Low Future Liver Remnant Volume, Including a Comparison with the Associating Liver Partition with Portal Vein Ligation for Staged Hepatectomy Approa. J Am Coll Surg. 2013; 217:126-33. doi:10.1016/j.jamcoll surg.2013.03.004. [ Links ]
53. Robles-Campos R, Brusadin R, López-Conesa A, López-López V, Navarro-Barrios Á, López-Espín JJ, et al. Long-Term Outcome After Conventional Two-Stage Hepatectomy Versus Tourniquet-ALPPS in Colorectal Liver Metastases: A Propensity Score Matching Analysis. World J Surg . 2019; 43:2281-9. doi:10.1007/s00268-019-05031-w. [ Links ]
54. D’Haese JG, Neumann J, Weniger M, Pratschke S, Björnsson B, Ar diles V, et al. Should ALPPS be Used for Liver Resection in Interme diate-Stage HCC? Ann Surg Oncol. 2016; 23:1335-43. doi:10.1245/s10434-015-5007-0. [ Links ]
55. Chan ACY, Poon RTP, Chan C, Lo CM. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg . 2016; 263:e14-6. doi:10.1097/SLA.0000000000001272. [ Links ]
56. Chan ACY, Chok K, Dai JWC, Lo CM. Impact of split completeness on future liver remnant hypertrophy in associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) in hepa tocellular carcinoma: Complete-ALPPS versus partial-ALPPS. Sur gery. 2017; 161:357-64. doi:10.1016/j.surg.2016.07.029. [ Links ]
57. Wang Z, Peng Y, Hu J, Wang X, Sun H, Sun J, et al. Associating Li ver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann Surg . 2020; 271:534-41. doi:10.1097/SLA.0000000000002942. [ Links ]
58. Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, et al. ALPPS Ver sus Portal Vein Embolization for Hepatitis-related Hepatocellu lar Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg . 2019; XX. doi:10.1097/SLA.0000000000003433. [ Links ]
59. Li J, Moustafa M, Meiners J, Stüben O, Izbicki J, Heumann A. ASO Author Reflections: Optimizing the Oncological Outcome for Lo cally Advanced Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020; 27:1385-6. doi:10.1245/s10434-020-08274-3. [ Links ]
60. Serenari M, Zanello M, Schadde E, Toschi E, Ratti F, Gringeri E, et al. Importance of primary indication and liver function between stages: results of a multicenter Italian audit of ALPPS 2012-2014. HPB . 2016; 18:419-27. doi:10.1016/j.hpb.2016.02.003. [ Links ]
61. Balci D. Pushing the Envelope in Perihiler Cholangiocellular carcinoma Surgery: TIPE-ALPPS. Ann Surg . 2018; 267:e21-2. doi:10.1097/SLA.0000000000002604. [ Links ]
62. Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernández-Alejandro R, et al. High mortality after ALPPS for peri hilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB . 2017; 19:381-7. doi:10.1016/j.hpb.2016.10.008. [ Links ]
63. Sakamoto Y, Matsumura M, Yamashita S, Ohkura N, Hasegawa K, Kokudo N. Partial TIPE ALPPS for Perihilar Cancer. Ann Surg . 2018; 267:e18-20. doi:10.1097/SLA.0000000000002484. [ Links ]
64. Chan A, Chung PHY, Poon RTP. Little girl who conquered the “ALPPS’’. World J Gastroenterol . 2014; 20:10208-11. doi:10.3748/wjg.v20.i29.10208. [ Links ]
65. Wiederkehr JC, Avilla SG, Mattos E, Coelho IM, Ledesma JA, Conceição AF, et al. Associating liver partition with portal vein liga tion and staged hepatectomy (ALPPS) for the treatment of liver tu mors in children. J Pediatr Surg. 2015; 50:1227-31. doi:10.1016/j.jpedsurg.2014.10.019. [ Links ]
Received: May 12, 2020; Accepted: September 22, 2020











 texto en
texto en