Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cirugía
versión On-line ISSN 2250-639X
Rev. argent. cir. vol.112 no.4 Cap. Fed. dic. 2020
http://dx.doi.org/10.25132/raac.v112.n4.1547.02
Articles
Recommendations for the return to scheduled diagnostic and therapeutic endoscopy during the COVID-19 pandemic
In the present setting, the Board of Directors defined that the recommendations and guidelines generated by Asociación Argentina de Cirugía during COVID-19 pandemic should be implemented considering the following aspects:
The responsibility in the decision making during the surgical act corresponds to the surgeon and to the regulations emanated from the Crisis Committee, according to the phase or stage of the pandemic (Spanish Association of Surgeons Classification) of each Health Institution and to the viral replication in the corresponding geographical area, ad-referendum of the recommendations of the National and Regional Health Authorities.
The Committee on Flexible Endoscopy of the AAC, which is permanently engaged in advising and assisting surgeons who perform gastrointestinal endoscopy during the SARS-Cov-2 pandemic, drew up the following recommendations for the return to the scheduled activity, based on the experiences shared by different international scientific societies and renowned national centers dedicated to flexible endoscopy.
Background
▪▪During the COVID-19 pandemic, all elective endoscopic procedures have been suspended worldwide to optimize resources, decrease health care worker exposure, and reduce circulation of patients.
▪▪The mitigation measures taken in Argentina have flattened the infection curve, extending the duration of the pandemic so that the return to elective endoscopies could be delayed indefinitely.
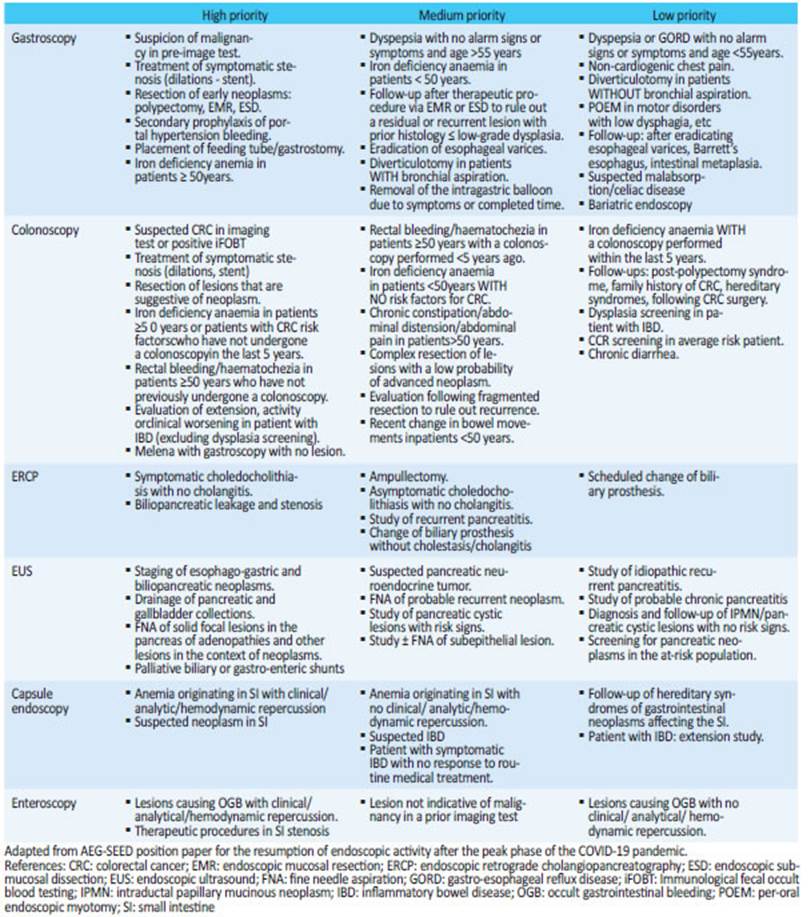
▪▪Currently, gastrointestinal endoscopy is limited to emergency or urgent procedures, and to patients with cancer (Table 1).
▪▪Because endoscopy is considered an aerosol-generating procedure and therefore poses a high risk for transmission of the SARS-CoV-2, the use of personal protective equipment (PPE) is recommended for all the procedures and all the staff.
Recommendations of the Committee on Flexible Endoscopy
▪▪As the different regions of the country present different epidemiological scenarios, the Committee of Flexible Endoscopy of the Asociación Argentina de Cirugía suggests adding a list of PRIORITY INDICATIONS for endoscopy to the indications of urgent, emergency and oncological procedures (Table 2). The aim of this list is to start the gradual return to routine endoscopy, rescheduling those endoscopies postponed during the pandemic. The indications of the elective procedures may be categorized in high priority, medium priority and low priority, based on the probability of finding clinically relevant lesions. We recommend performing high priority procedures first, followed by those with medium priority, leaving low priority procedures in the last place. This list is intended as a guide and the indication should be made based on medical judgment and the epidemiological situation of the region .
▪▪The following endoscopic procedures can be rescheduled: 1. Therapeutic procedures; 2. Endoscopy due to new-onset symptoms; 3. Follow-up or surveillance or previous conditions; 4. Screening for colorectal cancer (CRC). Therapeutic procedures are the cases that should be resolved most quickly to avoid a loss of opportunity, while surveillance or screening procedures on average-risk subjects should be carried out in the last place. It is recommended that the reason for rescheduling be reflected in the patient’s medical record, as well as a descriptive commentary justifying the level of priority including the indication, and to ensure proper communication between the various individuals involved in the process (attending physician, patient and endoscopist). This plan should be monitored regularly and modified if necessary.
▪▪Is recommended to ask all the patients to sign the Addendum to the Informed Consent timely proposed by Asociación Argentina de Cirugía for the procedures carried out during the pandemic.
▪▪It is advisable that the members of the endoscopy team sign a declaration of absence of symptoms suggestive of COVID-19 before entering the Endoscopy Unit (EU).
▪▪Screening for COVID-19 via telephone call in all the patients 24 hours before the procedure according to the definitions of the National Ministry of Health: fever (≥37.5ºC), cough, odynophagia, respiratory distress, recent anosmia/ageusia/disgeusia. Contact with confirmed cases of COVID-19 within the 14 days prior to symptom onset; residing or travel to an area (in the country or abroad) with community transmission anytime within the 14 days prior to symptom onset; residing or working in closed institutions; essential workers; or residing in slums or native people communities. The same questions must be repeated on the day of the study before the patient is admitted to the EU, controlling body temperature and evaluating the presence of gastrointestinal symptoms.
▪▪Screening for SARS-CoV-2 infection by RT-PCR, rapid antigen testing and serology test before endoscopy will not be considered a universal practice in our environment but could be performed in special situations. In addition to the risk posed by a false negative result for healthcare staff and other patients, the disadvantages associated with false positives should be considered: increased likelihood of contagion when passing through circuits established for patients with COVID-19, or isolation with delay of the endoscopy.
▪▪ It is recommended to delay elective cases that are suspected of COVID-19. As long as the epidemiological situation in the region entails a high risk of transmission or viral circulation, the rest will be carried out as if all patients were potentially infected. It is recommended for ALL healthcare staff involved in the performance of endoscopic procedures to wear PPE with high level of protection (Figure 1) in ALL the procedures.
▪▪It is necessary to establish protocols for the circulation of patients in the EU since they arrive and leave the room (Fig. 2) minimizing any unnecessary contact and the time spent in the unit. They should keep a distance between them of 1.5-2 meters . Patients should be provided with hospital clothing, surgical masks and hand hygiene products (soap and water or alcohol-based hand sanitizer) upon admission and discharge. Escorts SHOULD NOT enter the EU; in case of necessity, they must undergo the same screening as the patients. It is recommended to establish a separate circuit for patients with highly suspected or confirmed COVID-19.
▪▪The number of procedures must be reduced to achieve adequate social distancing and reduce exposure to infection among patients in the endoscopy unit and escorts in the waiting room.
▪▪Due to the need to implement additional health and safety measures, it is recommended to modify the time assigned to each endoscopy and the volume of procedures . The circulation of patients will be slower as a result of SARS-CoV-2 infection screening and hygiene measures. The activity of the health care staff will also be slowed down due to donning and doffing the PPE. In addition, additional time is necessary for cleaning, disinfecting and ventilating the endoscopy room each procedure.
▪▪ All the healthcare staff must know and follow the protocols used.
▪▪ It is convenient to perform endoscopies in rooms with negative pressure. If negative pressure is not available, the endoscopy room must be ventilated after the patients leave the room. In case of patients with suspected or confirmed COVID-19, the room must be ventilated for 1-3 hours , which is the time aerosols remain in the air. COVID-19 positive patients should be scheduled for the last appointment of the day.
▪▪ The endoscopies should be performed by expert operators.
▪▪The number of staff in the endoscopy room should be reduced to the minimum in order to reduce the risk of exposure and transmission.
▪▪Basic hygiene measures should be implemented and observed by the health care staff.
▪▪Due to the prevalence of the disease, and because endoscopy is an aerosol-generating procedure, high level personal protection equipment should be used in all the cases, including hermetic goggles, FFP3 or N95-99 respirators, face shield and fluid resistant gown. Three to six complete PPEs should be available for each procedure.
▪▪ All the staff must show compliance with donning and doffing protocols . There must be a clean zone separated from the endoscopy room for donning. Doffing is considered the critical step, with the greatest risk of inadvertent contamination. Donning and doffing should be supervised to reduce this risk.
▪▪It is recommended to remove PPE in an area of transition between the room ((contaminated area) and clean area. If this is not feasible, PPE can be doffed by the door to the endoscopy room, except for the goggles and N95 respirator which should be removed in the clean area.
▪▪It is advisable for oxygen therapy during sedation to be supplemented with nasal tubes at a flow of 5 L/min with a surgical mask above. To minimize the emission of droplets during upper gastrointestinal endoscopy, it is advisable to put a valve made with a glove finger on the endoscope port. Ensure perfect mask fit. If manual ventilation is required, place an HMEF filter between the mask and the Mapleson circuit. It is advisable not to insufflate air while the endoscope is in the oropharynx. The surgical mask is repositioned once the patient has recovered and maintains an oxygen saturation > 90%.
▪▪ Avoid taking biological samples when the clinical impact of the result is expected to be marginal. Carry out the processing of biological samples in line with the standardized biosecurity protocols for substances with a high infectivity capacity. Report when the sample was taken from a suspected or positive patient.
▪▪ It is advisable for the disinfection and re-processing of endoscopes and accessory devices to be carried out according to the usual regulations and protocols.
▪▪It is advisable to use single-use endoscopic accessories and NOT using it more than once.
▪▪It is recommended to apply protocols for the cleaning and disinfection of endoscopy rooms after each procedure , ensuring the disinfection of all the surfaces and devices that have come into contact with the patient or his/her secretions. The assignment of dedicated EU cleaning staff is recommended.
▪▪It is recommended for waste management to be carried in accordance with the local protocols for waste with high infectivity capacity.
▪▪It is advised to maintain a distance between people of 1.5-2 meters, implement basic hand hygiene measures and establish a separate patient circuit in the recovery rooms.
▪▪It is recommended to consider the implementation of follow-up programs by telephone contact for patients 7−15 days after the procedure to assess the onset of symptoms that are compatible with COVID-19. In case this occurs, the aim of this measure is the early identification of the staff members who have been in contact with that patient and to act in the chain of transmission, reducing the appearance of foci.
▪▪ In this way, we believe that timely return to the elective activity can take place safely.

Figure 1 Complete personal protection equipment (PPE3). Adapted from Position Statements on COVID-19: APSDE, ASGE and CDC 2020
Bibliografìa / References
Gupta S, Shahidi N, Gilroy N, et al. Proposal for the return to routi ne endoscopy during the COVID-19 pandemic. Gastrointest Endosc 2020 [ Links ]
Documento de Posicionamiento AEG-SEED para el reinicio de la ac tividad endoscópica tras la fase pico de la pandemia por COVID-19. Sociedad Española de Endoscopia Digestiva - Asociación Españo la de Gastroenterología. Publicado el 24/04/2020 www.wseed.org [ Links ]
JAG Endoscopy: Principles for restoration of Endoscopy Services fo llowing the COVID-19 pandemic. Royal College of Physicians - Joint Advisory Group on GI Endoscopy. Abril 2020 [ Links ]
Recomendaciones de la Asociación Tailandesa de Gastroenterolo gía. Position Statements on COVID-10: APSDE, ASGE and CDC 2020 www.thaitage.org [ Links ]
SIED: Recommendations for The Endoscopy Units during the Coro navirus infection Outbreack (COVID-19) versión 3.0, update as to April 14, 2020. www.siedonline.org [ Links ]
ASGE - American Society for Gastrointestinal Endoscopy. Gastroen terology professional Society Guidance on endoscopic procedures during the COVID 19 pandemic. www.asge.org [ Links ]
Procedimiento para el Reprocesamiento de Endoscopios. Ministerio de Salud de la Nación de la República Argentina. Anexo_5959196_1. Publicado el 02/04/2020 [ Links ]
Guía para procedimientos Endoscópicos y COVID-19 (versión 2). FA AAAR - Federación Argentina de Asociaciones. Anestesia, Analgesia y Reanimación. www.anestesia.or.ar [ Links ]
Chiu Ph, Ng S, Inoue H, et al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Di gestive Endoscopy (APSDE-COVID statements). Gut 2020; 69: 991- 6. [ Links ]
WEO Guidance to Endoscopists: Reopening of Endoscopy Services. The WEO COVID-19 Response Taskforce. www.worldendo.org [ Links ]
ASGE - American Society for Gastrointestinal Endoscopy. Guidance for Resuming GI Endoscopy and Practice Operations after the CO VID-19 pandemic. www.asge.org [ Links ]
Gralnek I, Hassan C, Beilenhoff U, et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pande mic. Endoscopy 2020; 52. [ Links ]
Marin-Gabriel J, Santiago E. Documento de Posicionamiento AEG-SEED para el reinicio de la actividad endoscópica tras la fase pico de la pandemia de COVID-19. Gastroenterol Hepatol 2020. https://doi.org/10.1016/j.gastrohep.2020.05.004 [ Links ]











 texto en
texto en