Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista argentina de cirugía
versión On-line ISSN 2250-639X
Rev. argent. cir. vol.113 no.1 Cap. Fed. abr. 2021
http://dx.doi.org/10.25132/raac.v113.n1.1529.ei
Articles
Extranodal lymphomas of the head and neck
1 División Cirugía Oncoló gica. Hospital de Clínicas José de San Martín. Universidad de Buenos Aires. Buenos Aires. Argentina.
Introduction
In the United States, 74 200 new cases of non- Hodgkin lymphoma were estimated to occur by 2019, which would represent 4.3% of cancer patients and 19 970 or 3.3% of all cancer deaths1.
According to the Instituto Nacional del Cáncer, 3405 new cases of non-Hodgkin lymphoma were diagnosed in Argentina in 2018, corresponding to 2.7% of all cancer patients in both sexes2. Extranodal lymphomas arise from sites other than lymph nodes and lymphatic organs with no or only minor nodal involvement. Approximately 30% of all lymphomas are extranodal3,4.
The head and neck region is the second most frequent site of extranodal lymphomas after the gastrointestinal tract. These tumors comprise a heterogeneous group of neoplasms with different types of presentation and aggressiveness3-7. The surgeon should know how to identify this entity among the different diagnosis of head and neck tumors, as surgical biopsy is necessary for the adequate diagnosis and to guide radiation therapy or systemic treatment.
The aim of this study was to describe the pathological, clinical and surgical characteristics of a consecutive series of patients undergoing surgery for ELHN.
Material and methods
We conducted a retrospective, descriptive and observational study in a tertiary university hospital. The electronic pathology records of 5489 patients operated on between June 2009 to June 2019 were reviewed, using the term “lymphoma” and the filters “extranodal” and “head and neck” locations. Patients operated on in other departments of our hospital and pediatric patients were excluded from the analysis.
Nineteen patients (0.35%) presented ELHN. The following variables of the clinical records were analyzed: age, sex, location, history of concomitant systemic diseases, pathology report and surgical technique.
The study was conducted following the ethical principles of the Declaration of Helsinki and revised in Tokyo. The clinical data were protected so as not to identify to whom they belong and not to be accessible to persons not bound by professional secrecy. All the patients signed an informed consent form approved by the Committee on Ethics of the institution.
Results
Mean age was 56 years (range: 17-84; SD 19.3) and 84% (n = 16) were women.
The location of the ELHN were the parotid gland (n =12, 63%), submaxillary gland (n = 3, 16%), salivary gland (n = 1, 5%), lacrimal gland (n = 1, 5%) and thyroid gland (n =1, 5%).
All the tumors were non-Hodgkin lymphomas with the following subtypes: mucosa associated lymphoid tissue (MALT) lymphoma (n = 9, 48%), follicular lymphoma (n = 4, 21%), large B-cell lymphoma (n = 3, 16%), small B-cell lymphoma (n = 1, 5%), mantle cell lymphoma (n = 1, 5%), and anaplastic lymphoma (n =1, 5%). Table 1 shows the distribution by organ.
Four patients (21%) presented Sjögren syndrome at the moment of the diagnosis; three were MALT lymphomas and one patient had follicular lymphoma.
There were no cases of nodal involvement and all corresponded to stage IE of the Lugano classification8,9.
Of the 12 patients with ELHN of the parotid gland, 7 presented heterogeneous masses with diffuse involvement of the gland and 5 had defined nodules without lymph node involvement. Only one patient presented facial paralysis at the moment of consultation.
The diagnosis was confirmed by imaging tests (musculoskeletal ultrasound, computed tomography (CT) scan or magnetic resonance imaging (MRI)] (Figures 1 and 2).
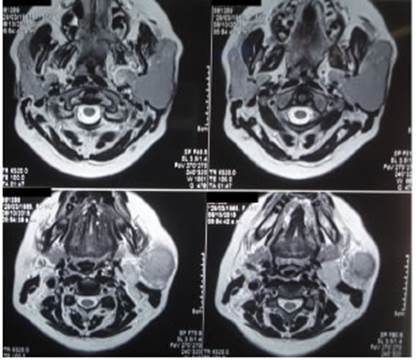
Figure 1 Magnetic resonance imaging without contrast agent in a patient with MALT lymphoma of the left parotid gland.
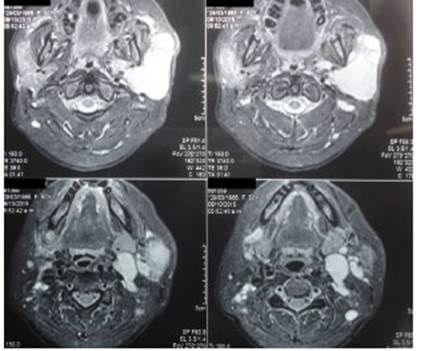
Figure 2 Magnetic resonance imaging with gadolinium-based contrast agent in a patient with MALT lymphoma of the left parotid gland.
All the patients underwent fine needle aspiration (FNA) biopsy but the results obtained were not conclusive and the diagnosis was only confirmed with the histological examination. The surgical procedures included 6 surgical biopsies of the parotid gland (due to probable lymphoma or unresectable malignant tumor), 5 superficial parotidectomies and 1 total parotidectomy (due to suspected benign or malignant tumor of the parotid gland).
In the 3 patients with lymphoma of the submaxillary gland, the FNA biopsy was not conclusive and the gland was resected due to clinical suspicion for malignancy. None of them presented clinical signs of marginal mandibular branch injury during the follow-up visit after surgery.
The cases localized in the minor salivary gland, lacrimal gland and thyroid gland were diagnosed with surgical biopsy. The tumor of the minor salivary gland was detected during the valuation of Sjögren syndrome. The surgical biopsy of the lacrimal gland tumor was performed due to suspicion for malignancy and impossibility to perform FNA biopsy. The diagnosis of lymphoma of the thyroid gland was suspected after 18FDG PET-CT scan10. After postoperative follow-up was completed, the patients continued treatment with the department of hematology.
Discussion
In order to define lymphomas and define the stage of the disease according to the Lugano classification, the lymph nodes, thymus, spleen and Waldeyer’s ring (tonsils, base of tongue and palate) are considered lymphatic organs and all the other tissues are extranodal sites3,4,8.
However, some authors consider that the Waldeyer’s ring is an extranodal organ and the most common site of ELHN5-7,11.
Most ELHN are non-Hodgkin lymphomas and the prevalence of Hodgkin disease in extranodal sites is very low (3.5%)12,13.
The present series does not include lymphomas originating on the Waldeyer’s ring and none of the cases corresponded to Hodgkin lymphomas.
In Chile, Peña et al. reported 1215 extranodal lymphomas (25%) in their series of 4907 non-Hodgkin lymphomas. Mean age was 59 years and 55% were women. The gastrointestinal tract was the most common location (37%), followed by the head and neck (24%) and skin (15%). The sites of origin were Waldeyer´s ring (28%), nasal cavity (25%), oral cavity (12%), thyroid gland (12%), major salivary glands (9%), lacrimal glands (8%), and paranasal sinuses (6%)7.
In China, the prevalence of extranodal (NK)/T-cell lymphoma is high, and 80% of cases occur in the head and neck, the Waldeyer’s ring, and paranasal sinuses, also known as “lethal midline granuloma”14.
Between 12 and 16% of ELHN are located in the salivary glands, representing between 2 and 5% of neoplasms of these glands; most are located in the parotid gland and 20% in the submaxillary and minor salivary glands11,15.
In our series, 63% were lymphomas of the parotid gland, probably because our center has great experience in treating this condition, and because there were no patients with tumors of the Waldeyer’s ring.
Lymphomas are more common in men. However, extranodal lymphomas of the salivary glands and thyroid glands are most common in women (80%)7,15, as in our series. The history of autoimmune diseases (which are more common in female patients) could justify this finding. The distribution by sex was similar to the one reported by other investigators5,14.
Primary lymphomas of the thyroid gland are rare, accounting for 5% of all thyroid neoplasms, and less than 3% of ELHN. Large B-cell lymphoma and MALT lymphoma are the most common types. Their clinical presentation is a rapidly growing euthyroid goiter with symptoms due compression of the adjacent organs (dysphagia, dysphonia, dyspnea)16,17.
MALT lymphoma of the thyroid gland mainly occurs in female patients with a history of Hashimoto’s thyroiditis18. Its association with a papillary carcinoma is rare but has been described19.
The imaging tests provide information about the local extension of the disease but do not have specific characteristics. Contrast-enhanced MRI describes tumor behavior after intravenous administration of gadolinium-based contrast agent. Benign neoplasms (such as pleomorphic adenomas) have been reported to exhibit late enhancement (greater than 150 seconds) after the administration of contrast agent, while malignant neoplasms (such as mucoepidermoid carcinomas) present early enhancement (before 150 seconds). The washout rate of the contrast agent is more rapid in benign tumors than in malignancies (washout rate > 30% versus < 30%, respectively). However, lymphomas of the salivary glands exhibit early enhancement after the administration of contrast agent (as carcinomas) and rapid washout (as adenomas)20. 18FDG PET-CT scan is useful for the diagnosis of Hodgkin lymphomas and diffuse large B-cell lymphomas. The Deauville score was published in France in 2009. This scale compares baseline 18FDG uptake in lymphomas with mediastinum uptake and liver uptake10.
Ultrasound-guided FNA biopsy is a useful tool for the diagnosis of many diseases of the head and neck. Nevertheless, the cytologic examination does not make a definite diagnosis in lymphoproliferative disorders. Ultrasound-guided core-needle biopsy (with Tru-Cut® biopsy device) could play a role in the diagnosis but has the limitation of the size of the lesion and the possibility of nerve injury, particularly in the parotid gland. In addition, the histopathological examination requires a sample of tissue large enough to perform other tests, as flow cytometry and cytogenetic analysis, which are necessary for a correct typification in non-Hodgkin’s lymphomas5.
In the case series here described, the use of diagnostic imaging tests varied depending on the availability of the tests. Both imaging tests and FNA biopsy made the diagnosis in few cases. In all the patients the diagnosis was confirmed by histopathological examination of the resected specimen, and the extent of the specimen varied according to the preoperative suspicion.
Autoimmune thyroid diseases and Sjögren syndrome may evolve into B-cell non-Hodgkin lymphomas of salivary glands and thyroid gland, with a prevalence of 0.5 and 5%, respectively21.
Autoimmune diseases produce states of chronic inflammation in which the persistent antigenic stimulation of the different pro-inflammatory cytokines, as IFN-γ and IL-10, promotes the activation and proliferation of B-cells22. There is also an association between autoimmunity and lymphatic proliferation in the histological level. In patients with autoimmune diseases, the lymphocytic infiltration of the glandular tissues predominantly by activated CD4+ T-cells promotes the development of germinal center-like structures where B cells are activated21. Patients with Sjögren syndrome are at higher risk of lymphoma than patients with other autoimmune diseases (7- and 4-fold in systemic lupus erythematosus and rheumatoid arthritis patients, respectively) and healthy individuals (>10-fold)22. Only a few of these will occur in the head and neck.
The prevalence of Sjögren syndrome in patients with lymphomas of the salivary gland is 18%23. However, there is a group of patients in whom the diagnosis of Sjögren syndrome is made after the diagnosis of the lymphoproliferative disease. Therefore, lymphoma of the salivary gland should be ruled out in all patients with Sjögren syndrome24.
Sjögren syndrome and autoimmune thyroid diseases are characterized by a wide spectrum of genetic and molecular abnormalities that culminate in uncontrolled B-cell proliferation and neoplastic transformation21.
In patients with acquired immunodeficiency syndrome, non-Hodgkin lymphoma represents the second most common malignancy after Kaposi sarcoma. HIV infection increases the risk of developing non-Hodgkin lymphoma by 100 to 200 times25. In Argentina, Corti et al. published a series of 34 patients with AIDS and ELHN; 75% were men and mean age was 39 years. The gingiva and the hard palate were the most common site of the lesions (62%), followed by the upper and lower maxilla (26%), the skin and soft tissue (21%) salivary glands (13%), and Waldeyer’s ring25.
A recent meta-analysis identified hepatitis C virus (HCV) as a risk factor for the development of extranodal orbital lymphoma26.
The histological subtype and the primary organ are the most important prognostic factors in extranodal lymphomas, due to the differences in the natural history of the disease and to the organ-specific therapy. Complete follow-up was not possible in our series to assess long-term outcomes since the definitive treatment was carried out in other institutions in many cases.
Peña et al. reported an overall survival of 63%, 50% and 38% at 5, 10 and 15 years, respectively, with a mean follow-up of 9.6 years7.
In Taiwan, Chi et al. described of 68% and 57.8% at 5 and 20 years, respectively, with disease-free survival of 53.6% and 49.3%, respectively.
The findings of our series let us conclude the ELHN are rare neoplasms occurring in middle-aged patients, particularly in women. The clinical picture can coexist with other regional diseases, and imaging tests or FNA biopsy fail to make the preoperative diagnosis. Thus, head and neck surgeons must think of extranodal lymphomas in order to perform the required surgical intervention for an appropriate diagnosis and treatment.
Referencias bibliográficas /References
1. Siegel RL, Miller KD, Ahmedin J. Cancer Statistics 2019. CA Cancer J Clin. 2019; 69(1):7-34.doi:10.3322/caac.21551. Epub 2019 Jan 8. https://seer.cancer.gov/statfacts/html/nhl.html [ Links ]
2. Instituto Nacional del Cáncer de Argentina. Estadísticas. Incidencia. Fecha de consulta: 18 de mayo de 2020. https://www.argentina.gob.ar/salud/instituto-nacional-del-cancer/estadisticas/incidencia [ Links ]
3. d’Amore F, Christensen BE, Brincker H, et al. Clinicopathological features and prognostic factors in extranodal non-Hodgkin lymphomas. Danish LYFO Study Group. Eur J Cancer. 1991; 27(10):1201-8. [ Links ]
4. Vannata B, Zucca E. Primary extranodal B-cell lymphoma: current concepts and treatment strategies. Chin Clin Oncol. 2015; 4(1):10. doi: 10.3978/j.issn.2304-3865.2014.12.01. [ Links ]
5. Picard A, Cardinne C, Denoux Y, Wagner I, Chabolle F, Bach CA. Extranodal lymphoma of the head and neck: A 67-case series. Eur Ann Otorhinolaryngol Head Neck Dis. 2015; 132(2):71-5. [ Links ]
6. Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005; 9(6):340-50. [ Links ]
7. Peña C, Russo M, Martínez V, Cabrera ME. Extranodal lymphomas in the public health system in Chile: Analysis of 1251 patients from the National Adult Cancer Program. Hematol Oncol. 2019; 37:47-53. [ Links ]
8. Zelenetz AD, Jaffe ES, Advani RH, et al. Hodgkin and Non-Hodgkin lymphomas. In: AJCC Cancer Staging Manual. 8th edition. New York: Springer; 2017. Chapter 79, pp. 937-58. [ Links ]
9. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32(27):3059-68. [ Links ]
10. Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009; 50(8):1257-60. [ Links ]
11. Cabeçadas J, Martínez D, Andreasen S et al. Lymphomas of the head and neck region: an update. Virchows Arch. 2019; 474:649- 65. [ Links ]
12. Iyengar P, Mazloom A, Shihadeh F, Berjawi G, Dabaja B. Hodgkin lymphoma involving extranodal head and neck sites. Cancer. 2010; 116:3825-9. [ Links ]
13. Feinstein AJ, Ciarleglio MM, Cong X, Otremba MD, Judson BL. Parotid gland lymphoma: prognostic analysis of 2140 patients. Laryngoscope. 2013 y; 123 (5):1199-203. [ Links ]
14. Tse E, Kwong YL. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol . 2017; 10:1-13. [ Links ]
15. Jamal B. Treatment of parotid non-Hodgkin lymphoma: a meta-analysis. J Glob Oncol. 2018; 4:1-6. doi: 10.1200/JGO.17.00071. [ Links ]
16. Walsh S, Lowery AJ, Evoy D, McDermott EW, Prichard RS. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. Oncologist. 2013; 18(9):994-1003. [ Links ]
17. Thieblemont C, Mayer A, Domontet C, et al. Primary thyroid lymphoma is a heterogeneous disease. J Clin Endocrinol Metab. 2002; 87(1):105-11. [ Links ]
18. Karvounis E, Kappas I, Angelousi A, Makris GM, Kassi E. Mucosa-associated lymphoid tissue lymphoma of the thyroid gland: a systematic review of the literature. Eur Thyroid J. 2020; 9:11- 8. [ Links ]
19. Lan X-B, Cao J, Zhu X-H, et al. Concomitant papillary thyroid carcinoma and mucosa-associated lymphoid tissue thyroid lymphoma in the setting of Hashimoto thyroiditis. Int J Clin Exp Pathol. 2018; 11:3076-83. [ Links ]
20. Lam PD et al. Differentiating benign and malignant salivary gland tumours: Diagnostic criteria and the accuracy of dynamic contrast-enhanced MRI with high temporal resolution. Br J Radiol. 2015; 88:8-12. [ Links ]
21. Baldini C, Ferro F, Mosca M, Fallahi P, Antonelli A. The association of Sjögren syndrome and autoimmune disorders. Front Endocrinol (Lausanne). 2018; 9:121. doi: 10: 10.3389/fendo.2018.0021. eCollection 2018. [ Links ]
22. Retamozo S, Brito-Zerón P, Ramos-Casals M. Prognostic markers of lymphoma development in primary Sjögren syndrome. Lupus. 2019; 28:923-36. [ Links ]
23. Travaglino A, Giordano C, Pace M, et al. Sjögren Syndrome in Primary Salivary Gland Lymphoma. A Systematic Review and Meta- Analysis. Am J Clin Pathol. 2020; 153(6):719-24. doi: 10.1093/ajcp/aqaa005. [ Links ]
24. Vasaitis L, Nordmark G, Theander E, et al. Comparison of patients with and without pre-existing lymphoma at diagnosis of primary Sjögren’s syndrome. Scand J Rheumatol. 2019; 48(3):207- 12. [ Links ]
25. Corti M, Villafañe M, Bistmans A, Narbaitz M, Gilardi L. Primary extranodal non-hodgkin lymphoma of the head and neck in patients with acquired immunodeficiency syndrome: A clinicopathologic study of 24 patients in a single hospital of infectious diseases in Argentina. Int Arch Otorhinolaryngol. 2014; 18(3):260-5. [ Links ]
26. Travaglino A, Varricchio S, Pace M, et al. Hepatitis C virus in MALT-lymphoma of the ocular adnexa. Pathol Res Pract. 2020; 216(4):152864. doi:10.1016/j.prp.2020.152864. [ Links ]
27. Chi H-S, Lee K-W, Chiang F-Y, et al. Head and neck extranodal lymphoma in a single institute: a 17-year retrospective analysis. Kaohsiung J Med Sci. 2012; 28(8):435-41. doi: 10.1016/j.kjms.2012.02.014. Epub 2012 Apr 22. [ Links ]
Received: June 17, 2020; Accepted: October 14, 2020











 texto en
texto en 



