KEY POINTS
Current knowledge
• The use of sodium dichloroacetate (DCA) in cancer treatment has been explored, es pecially because of its modulating activity on cellular metabolism after the inhibition of pyruvate dehydrogenase kinase. Howe ver, the lack of controlled clinical studies has left doubts about its effectiveness and safety.
Contribution of the article to current knowledge
• Despite promising preclinical evidence, results from clinical studies and case re ports show a lack of conclusive evidence on the efficacy and safety of DCA in cancer treatment. The heterogeneity of the studies and the presence of adverse events, such as peripheral neuropathy, highlight the need for additional controlled investigations.
In recent years, drug repurposing (DR) has become a valuable strategy for identifying new therapeutic uses for approved drugs. This ap proach is particularly appealing given the high cost and lengthy timelines associated with tra ditional drug discovery processes. In oncology, DR has a long history dating back to the devel opment of the first chemotherapy drugs, chlorambucil (Leukeran®) and busulfan (Myleran®), which were derived from ‘mustard gas’ used during the I World War. Advances in our under standing of the chemical structures and mecha nisms of action of drugs have made it possible to repurpose drugs for different types of diseases, even when they were not originally designed for those indications1-4. In cancer research, many old drugs have been identified as possible useful by the Repurposing Drugs in Oncology project (ReDO)5, an international collaboration focus ing on the potential use of approved non-cancer drugs as a source of new cancer therapeutics.
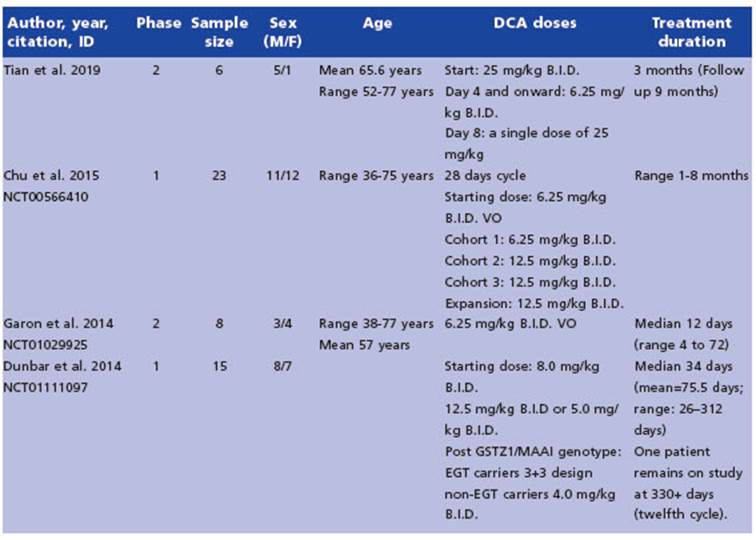
Particularly, the metabolism-oriented ap proach is a promising field for cancer therapy. Typically, tumor cells increased glucose uptake to generate lactate even in the presence of oxygen, a phenomenon known as the Warburg effect or aerobic glycolysis6 (Fig. 1). The high generation of energy with high ATP: glucose ratio occurring in normal cells, through the mitochondrial oxida tive phosphorylation (OXPHOS), makes a switch in cancer cells to the less efficient process of aerobic glycolysis, characterized by low ATP: glu cose ratio, inhibition of pyruvate dehydrogenase (PDH), the enzyme that catalyzes the oxidative conversion of pyruvate into acetyl coenzyme A in mitochondria, lactic acid fermentation and low energy production7. The excessive glycolysis and consequent overproduction of lactate leads to a state of metabolic acidosis in the tumor mi croenvironment. Lactate, derived from glycoly sis, is absorbed by surrounding cells to support tumor growth and to inhibit mechanisms of cell death, such as apoptosis. This process promotes tumor progression and is considered a hallmark of cancer.
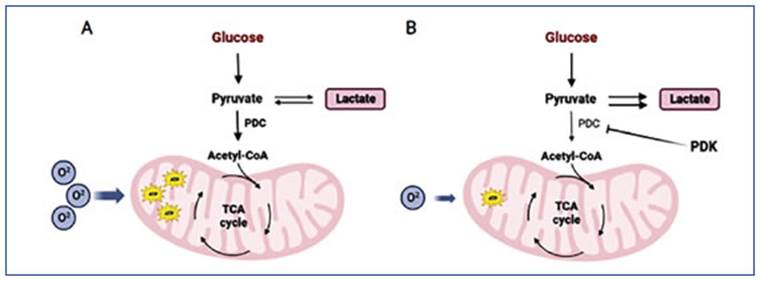
Figure 1 Warburg effect. Glucose metabolism in a normal cell (A) and a cancer cell (B). Figure created in Bio Render
Sodium dichloroacetate (DCA), used to treat congenital lactic acidosis, can shift the tumor metabolism by reactivating the oxidative phos phorylation in the mitochondria. DCA works by inhibiting PDK, an enzyme that normally inhib its PDH8. By blocking PDK, DCA activates PDH and stimulates the mitochondrial oxidation of pyruvate. This disrupts the metabolic advantage of cancer cells and can potentially slow down their growth. Additionally, DCA not only inhib its the growth and spread of tumors by reducing lactate production and counteracting the acidic microenvironment, but it also induces organelle remodeling by supplying pyruvate to the mito chondria. This leads to increased release of cytochrome c and other factors that promote apop tosis, as well as positive regulation of ROS levels, ultimately reducing cancer cell viability9 (Fig. 2). Based on the metabolic modulation caused by DCA, the antitumor capacity of this compound has been studied, aiming to be used in the treat ment of cancer10-13.
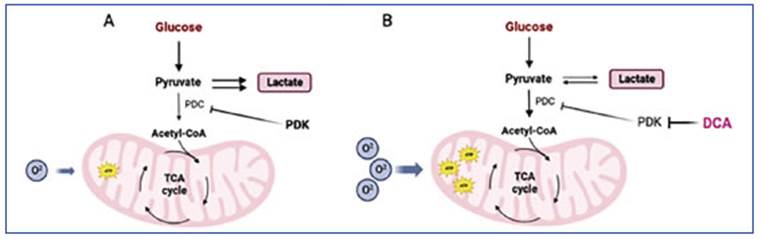
Figure 2 DCA mechanism of action. The altered cellular metabolism found in cancer cells (A) is normalized by DCA through the inhibition of PDK (B). Figure created in Bio Render
Numerous studies have been performed in cell lines and animal models to unveil its efficacy as an antitumor drug14-19. Even so, the lack of con trolled clinical studies makes the safety profile a concern. Making allowance for the aim of this work, we decided to set out what is known about the use of DCA for the treatment of cancer.
Therefore, this scoping review aimed to ex plore the existing scientific literature to pro vide an overview of the use of DCA (any dose, frequency, or route of administration) in adults with cancer.
Methods
This review was carried out following the methodolo gical guidance from The Joanna Briggs Institute (JBI) ma nual for Evidence Synthesis and was reported using the PRISMA for Scoping Reviews extension. Our protocol was registered at the medRxiv database20.
This review was conducted in 5 stages: identifying the research question, identifying relevant studies, study selection, charting the data, and collating, summarizing and reporting the results.
For the first stage, we set the question: What is known in the existing literature about the use of DCA for the treatment of cancer in adult patients? To identify relevant studies, we searched in MEDLINE/PubMed and LILACS da tabases for potentially relevant records on 1 March 2023. The search strategy included Medical Subject Headings (MeSH) terms, and their relevant synonyms joined with Boolean operators, and was the following: (Dichloroace tic Acid OR “dichloroacetate sodium” OR dichloroaceta te OR “sodium dichloroacetate”) AND adult AND (cancer OR neoplasms). Searches were not restricted by publica tion date, place, or type of study. EPISTEMONIKOS, and Cochrane Library databases were explored on 1 March 2023 to identify systematic reviews and meta-analyses. Additional studies were added by scanning references of relevant articles.
The studies were identified based on the eligibility criteria: All types of study design were included, publi cations reporting on adult patients diagnosed with any type of cancer, publications reporting dichloroacetate so dium treatment in combination or not with other drugs, publications written in English, Spanish, Portuguese, Ita lian, and French. We excluded publications reporting on children or pregnant women, and studies on cell lines or animal models.
After removing duplicates, two researchers (CB and RPM) screened the results at the title/abstract level and second at the full-text level independently. Discrepancies were resolved by consensus between the two reviewers.
Data were extracted by two researchers (CB and RPM), and disagreements were resolved by consensus. Extrac ted data included study characteristics, study design, participant population, condition, DCA composition, DCA doses, DCA route of administration, treatment duration, concomitant treatment, follow up; adverse events repor ted, and outcome measures.
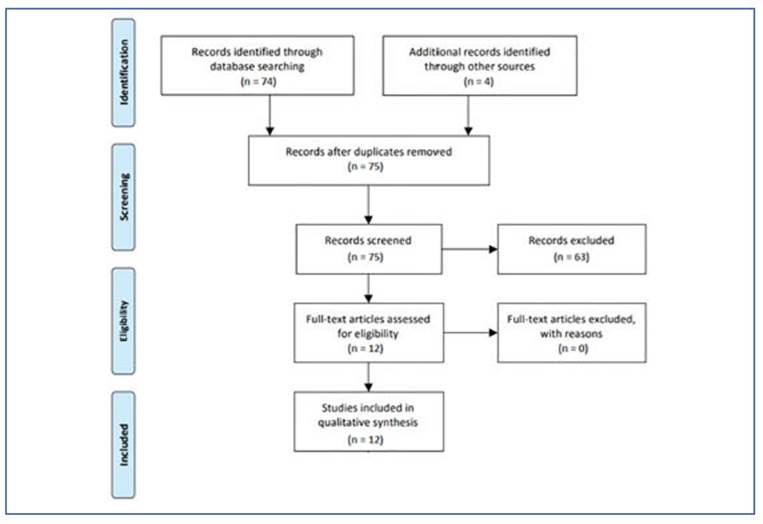
Finally, the PRISMA flowchart was used to present the study selection process21. The data from each article was presented in descriptive form and quantitative results were presented as appropriate in a table form.
Results
Study selection
A total of 52 scientific articles were identified through searches of MEDLINE/PubMed and LI LACS, and 3 articles were found through search es of Epistemonikos and The Cochrane Library. Additionally, 22 registers were identified from the ClinicalTrial database. All searches were con ducted on 01/03/2023. Four additional records were identified by searching references (Khan et al. 201622 and Khan et al. 201723) or by connecting with authors (Khan et al. 2013 and Khan et al. 2014). The titles and abstracts were assessed for their relevance to the review based on the eligi bility criteria, where 63 citations were excluded, resulting in 12 citations for full text review. One citation was excluded because full text was not available (Figure 3).
Characteristics of included studies
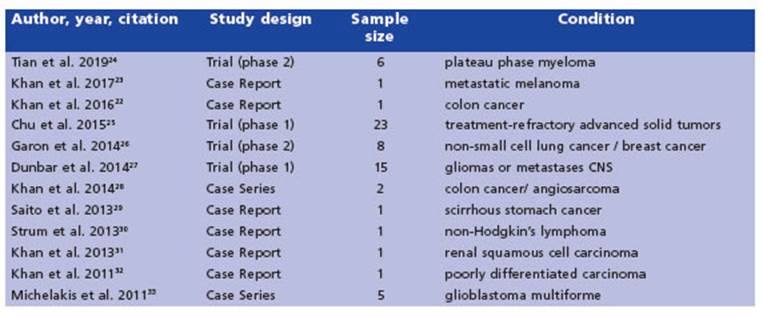
The 12 included studies were published be tween 2010 and 2019; seven studies were from Canada, three were from United States of Amer ica, one from Australia, and one from Japan. Study designs were clinical trials (n = 4), case re port (n=6), and case series (n = 2) (Table 1).
Case reports
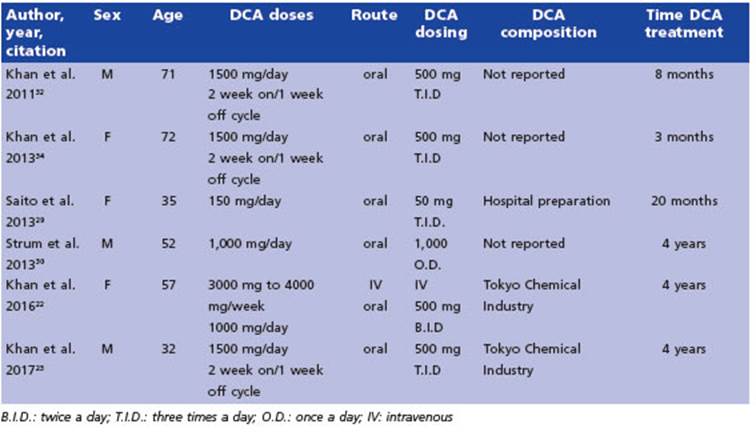
We reviewed six case reports that reported varying levels of efficacy. The most relevant characteristics are summarized in Table 2.
The first report we came across detailed the use of DCA therapy for pain reduction in a pa tient with carcinoma metastasis in their leg (Khan et al. 2011). The authors noted a significant decrease in tumor pain without any adverse events over a period of 20 months of treatment32.
In a subsequent study, Saito et al.29 described the case of a patient diagnosed with advanced gastric cancer who received a combination treatment of 5-aminolevulinic acid (ALA), hyper thermotherapy, immunotherapy, chemotherapy and DCA. The patient survived for one year and seven months, and no adverse events related to DCA treatment were reported. In that same year, Strum et al30 published a case report of a patient with non-Hodgkin’s lymphoma who began a DCA regimen, along with high caffeine supple ments and vitamins, on their own. The authors observed full remission of the patient’s condi tion, with no reported adverse events except for mild peripheral neuropathy after 4 years of treatment.
In 2013, Khan et al.31 reported on a 72-year-old woman with renal cancer who was treated with DCA for 3 months before experiencing symp toms of peripheral neuropathy. A follow-up ex amination showed no evidence of recurrence 5 years after the start of DCA treatment.
In a case study reported by Khan et al. in 201622, a woman with stage IV colorectal cancer received intravenous DCA therapy along with chemotherapy, vitamin D and C, and natural supplements to mitigate the risk of DCA side ef fects. Mild post-infusion sedation was the only side effect noted at the higher DCA dose (4000 mg i.v. (66 mg/kg)). After about 6 months of treatment, the patient discontinued chemother apy (Folfiti - Bevacizumab) but continued DCA therapy at a higher dose of 4500 mg i.v. weekly, which did not result in any significant changes in the disease but caused mild numbness of the fingers and toes and asymptomatic liver en zyme elevations - both of which were diagnosed as DCA side effects. The DCA therapy was inter rupted for 3 months to allow the resolution of the side effects. During this period, only natu ral therapies were given, and the patient’s mild DCA neuropathy resolved while liver enzymes began to improve. Oral DCA therapy (500 mg (8.2 mg/kg) twice a day) was later initiated along with neuroprotective supplements. The attempt to increase the dose to 500 mg three times a day was not possible due to adverse events (signifi cant asymptomatic liver enzyme elevation and increase in neuropathy) that resolved after a brief interruption, with the patient returning to the original treatment schedule. After almost 4 years of therapy, CT scans revealed stable dis ease.
In a study by Khan et al. in 201723, a male pa tient with metastatic melanoma was treated with oral DCA in a 2-week on, 1-week off cycle of 500 mg taken 3 times per day (17 mg/kg per day) for 4 years. To minimize the risk of DCA side effects, the patient was also prescribed three natural medications: oral acetyl L-carnitine, benfotiamine, and R-alpha lipoic acid. During the periods when the patient was taking DCA, he experienced a slight reduction in sensation in his fingertips and toes and had slightly reduced ability to concentrate. These mild symp toms were attributed to DCA-related neuropa thy, and they resolved during the weeks when the patient was not taking DCA. Despite these mild side effects, the patient remained stable and was able to work full-time.
Case Series
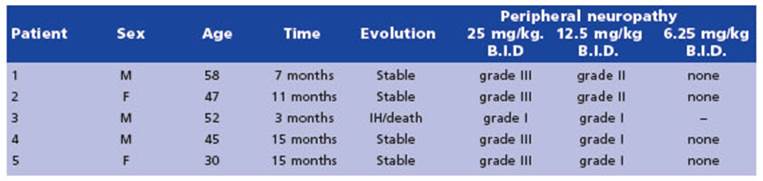
Michelakis et al. (2010)33 conducted a case series involving five patients diagnosed with glioblastoma multiforme who were treated with DCA. The treatment regimen involved an initial oral dose of 12.5 mg/kg twice a day for a month, which was then increased to 25 mg/kg twice a day. In cases where dose-limiting toxic ity occurred, a dose de-escalation protocol was followed, whereby the dose was reduced by 50%. The patients were monitored for up to 15 months, during which the only apparent adverse event was peripheral neuropathy. The degree of peripheral neuropathy varied among patients and was dose-dependent but reversible. When the dose was decreased to 6.25 mg/kg twice a day, none of the patients had clinically signifi cant peripheral neuropathy. At month 15 of DCA therapy, all patients except one were clinically stable, and at month 18, they were alive and without adverse events (Table 3).
Later, Khan et al.28 reported a case series of 3 patients with advanced cancer of which we analyzed only the two adult patients included. Both were treated with naturopathic treatment, vitamins and, intravenous DCA, and favorable responses were observed. The first patient, with a metastatic colon cancer, received 3 doses of DCA (3000 mg, 3500 mg, and 3700 mg). After the second infusion, the patient noted chills, sweats, and fatigue, and after the third one, he experi enced fatigue and malaise whereby he decided to stop the treatment. A month later it seemed to show tumor stability. The second patient had a metastatic angiosarcoma and underwent treatment with intravenous DCA, starting at a dose of 3000 mg per week and gradually in creasing to 5000 mg twice per week over a period of two weeks. In addition, the patient received concurrent radiotherapy for a spine metastasis. After eight months of treatment, the patient re mained clinically stable and did not experience any adverse events. However, the patient decid ed to discontinue the treatment and seek other alternative therapies.
Trials
The review consisted of four trials, two of which were Phase 1 trials that assessed the safety and tolerability of DCA (Chu et al. 201525 and Dunbar et al. 201427), and two Phase 2 trials principally assessing response to treatment (Tian et al. 201924 and Garon et al. 201426). All the trials utilized a commercial formulation of DCA, provided by TCI America. Except for Tian et al. 2019, the others used DCA as the only treatment (Table 4).
Chu et al. conducted a phase 1 study involving 23 patients, of whom only 3 were able to com plete the third cycle of DCA with stable disease. Ten patients did not finish the first cycle, with 7 experiencing disease progression and 3 with drawing consent due to adverse events. Fatigue (34.8%), neuropathy (34.8%), and nausea (17.4%) were the most reported adverse events. Grade 3 toxicities were predominantly fatigue (17.4%) and neuropathy (13.0%). Importantly, neuropa thy was reversible in most cases. However, one patient developed grade 3 neuropathy after re ceiving 17 doses of DCA, which did not resolve after 14 days of stopping DCA. This patient also had clinically progressive disease at the time of stopping therapy. While 8 of 23 patients had stable disease, most of the cohort was unable to continue treatment for this duration.
The other Phase 1 study was carried out by Dunbar et al. They included 15 individuals, but due to patient withdrawals, only eight patients were included in the final analysis. Of these eight subjects, the median duration of DCA treatment was 34 days (range 26-312 days), and all were clinically and radiographically stable at the end of the fourth week. At the time of result submission, three patients were still alive, while five had died. Two patients in the study reported grade 1 neuropathy, leading to a dose adjustment in one of them who was taking 6.25 mg/kg B.I.D. Another patient withdrew from the study due to the worsening of pre-existing gait abnormalities, which improved after the patient stopped taking the drug. Additional grade 1 and 2 adverse events were also reported. It is impor tant to note that all the patients were genotyped based on haplotype variation in the glutathione transferase zeta 1/maleylacetoacetate isomer ase (GSTZ1/MAAI) gene to determine dosing.
In addition, two Phase 2 trials were included. Tian et al. reported in a group with patients with plateau phase myeloma, that peripheral neu ropathy was the only significant side effect ob served during DCA treatment, which was only seen after chronic use and it was reversible. Fur thermore, five out of six patients who participat ed in the trial had preexisting neuropathy, and the treatment did not greatly exacerbate their condition. The researchers concluded that the serum DCA concentrations achieved were pos sibly not sufficient to affect tumor development.
In another Phase 2 trial, Garon et al. studied eight patients with non-small cell lung cancer. All of them experienced mainly low-grade ad verse events. None of the patients required a dose reduction, but three of them had their dos es delayed. Two patients died 4 and 7 days after the treatment began. Due to the lack of observed clinical benefits and the two deaths, it was de cided to close the study.
Finally, from the records identified from Clini cal Trial database, NCT05120284 is currently re cruiting patients with Glioblastoma Multiforme, with an estimated completion date of 2025. NCT00540176, have not been published although it was completed in 2014, and NCT00703859, was discontinued before enrolling its first partici pant.
Discussion
Drug repurposing offers a cost-effective ap proach to the battle against diseases such as cancer. One of the key advantages of this strategy is that it often takes advantage of drugs whose safety profiles are already well established. In this sense, many drugs are being evaluated to be repositioned.
Dichloroacetate, also known as DCA, is a drug that inhibits a crucial enzyme in the glucose metabolic pathway and has been tested in clinical trials for metabolic disorders. Recently, there has been a growing proposal to utilize this mecha nism to counteract the “Warburg effect” observed in cancer cells. As a result, scientists have started using it in animal models and in vitro assays, fol lowed by its application in human studies. DCA has also emerged as a potential therapeutic op tion to treat endometriosis, representing its lat est application in the repurposing field. However, despite being a relatively old drug, there is a need to understand its safety profile. In this work, we reviewed the existing scientific literature on the use of DCA in adult patients with cancer to evalu ate mainly the safety profile and efficacy. To our knowledge, this is the first review on the use of DCA in cancer treatment.
We included 12 studies with 65 patients. Six studies were case reports, two were case se ries, and four were early-stage clinical trials. It is important to highlight that most of them do not have a controlled methodology. Besides, there is a high degree of heterogeneity between them. The most common tumor was glioblas toma (15 patients), followed by lung cancer (7 patients) and myeloma (6 patients). Other can cers reported were gastrointestinal tumors, melanoma, angiosarcoma, breast cancer, non- Hodgkin’s lymphoma, and renal carcinoma. Finally, 24 patients from 2 studies had treatment-refractory or poorly differentiated solid tumors. In addition, the length of the treatment was di verse. Also, DCA was used alone or in combina tion with other drugs, and some of the studies reported a very short time of use, which makes it difficult to assess the efficacy of the treat ment. However, Phase 2 clinical trials did not demonstrate effectiveness, and they were not followed by Phase 3 studies. In fact, one of the Phase 2 studies included was terminated early due to lack of efficacy.
DCA doses were similar in most of the studies (6,25 mg/kg and 12,5 mg/kg BID, when adminis tered per os) but not all of them came from com mercial formulations.
The doses used were variable, not only the ini tial doses, but also the treatment cycle schemes, loading and maintenance. Moreover, commercial formulations were not used in all the cases. The most widely used average dose is 6.5 mg/kg BID.
Finally, regarding adverse effects, it is well known that chronic use of DCA causes reversible peripheral neuropathy, among other unwanted events. This happens because of the accumula tion of DCA due to the inactivation of GSTZ1-1. The most frequent adverse events in the evalu ated studies were asthenia, reversible neuro logic events like peripheral neuropathy, gait dis turbances, ataxia, hypersomnolence, increase in liver enzymes, abdominal pain, edema, nausea, and vomiting. In general, toxicity was described as dose dependent. In several cases, the loss of follow-up made it impossible to determine the reversibility of the neurologic events, and many patients withdrew their consent to participate in the study. Furthermore, the Phase 2 trial de veloped by Garon et al., was prematurely closed due to patient´s safety criteria, considering that the use of DCA caused two early patients’ death. The clearance of DCA mainly depends on the GSTZ1-1 enzyme, which function can alter the drug metabolism. The GSTZ1-1 gene exhib its polymorphisms that result in slow or rapid metabolizer phenotypes34. This is an important finding that may explain, at least in part, the variability in the response to DCA treatment in different patients and diseases.
This study has limitations. Assessing the ef fectiveness has been challenging due to the pa tients and studies characteristics, and the ab sence of a comparator. Also, there are questions about the general quality and reliability of the findings because most of the studies included in the analysis were case reports or case series.
To summarize, even though numerous stud ies have been published in animal models on the use of DCA to treat cancer, there are not enough publications on its controlled use in humans. We conclude that there is insufficient evidence to state neither the effectiveness nor the safety of DCA treatment in cancer patients.