INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has shown its benefit in high-risk or inoperable patients, 1,2 those at intermediate risk 3,4 and also in low-risk patients. 5,6
According to different analyses, the presence of mild paravalvular regurgitation (PVR) occurs in 20% to 40% of the cases, 7 while moderate or severe PVR in about 3% to 12% 2 and has been associated with higher mortality rates. 8,9 Although the incidence of PVR has decreased with the learning curve of the operators and the new devices, it represents a complication that should be avoided or managed during the procedure.
Another issue with self-expandable valves is the need for implantation of a permanent pacemaker (PPM), with an incidence of about 17%-30% in large, randomized studies. 2-6
To overcome these limitations, higher implant of percutaneous aortic valves using a view where the right and left cusps are overlapped (Cusp-overlap, COVL) is a technique developed to reduce contact with the membranous septum and conduction system and thereby reduce the need for PPM. 10
This strategy has demonstrated a significant decrease in the need for PPM in some observational series, 11-13 but the impact on the incidence of perivalvular leaks leading to moderate or severe regurgitation has not been clearly evaluated.
METHODS
We analyzed 206 consecutive patients undergoing TAVI with self-expandable valves between August 2019 and May 2022. The conventional technique (CON) was used in the first 101 patients (49%) and the COVL strategy in the subsequent 105 (51%).
Patients with previous surgical bioprosthetic valves, prior PPM, bicuspid aortic valve or pure or predominant aortic regurgitation as indication of TAVI or implant of a balloon-expandable valve were excluded from the study.
The primary endpoint (PEP) was the presence of moderate or severe PVR at 30 days, defined according to the criteria of the VARC-3. 14 The incidence of the following events was also analyzed: all-cause mortality, acute myocardial infarction (MI), acute coronary artery obstruction due to TAVI, stroke, major bleeding (as defined by VARC), vascular complication, emergency cardiac surgery, reintervention, need for PPM implantation, and new-onset left bundle branch block (LBBB) persistent at 30 days.
Implant success (IS) was defined as adequate valve implant with a residual gradient <10 mm Hg at the end of the procedure in the absence of severe regurgitation, and clinical success (CS) as IS in the absence of death, acute MI, stroke, reintervention or urgent valve surgery.
All the patients were evaluated by the hospital “Valve Heart Team”, with Doppler-echocardiography, coronary angiography with aortography, and a multi-slice contrast-enhanced computed tomography angiography with 3D reconstruction of the aortic valve, thoracic aorta, abdominal aorta, and subclavian, iliac and femoral arteries. An electrocardiogram (ECG) was recorded before TAVI, at 24 hours and at 30 days. Color Doppler-echocardiography was performed before TAVI, immediately after and at 30 days.
Anesthesia with conscious sedation was used, except for those in whom a percutaneous femoral access was not used and thus required general anesthesia. All patients received dual antiplatelet therapy with aspirin and clopidogrel; in those patients receiving oral anticoagulants for any other reason, only clopidogrel was indicated.
Anticoagulation with heparin 100 U/Kg was used during the procedure, with values controlled during its course.
In patients receiving implants with the CON strategy, the valve was positioned and implanted using the coplanar 3-cusp projection according to the computed tomography angiography and usually corrected by the left anterior oblique slightly cranial angiographic projection. The implantation was performed having as target an implant depth of ap proximately 2-4 mm below the aortic annulus under high stimulation frequency (120 bpm) with temporary pacing, at the operator’s discretion.
In those undergoing the COVL strategy, the computed tomography angiography was previously analyzed in detail, identifying the projection where there was right and left cusp overlap, in opposition to the non-coronary cusp, and this was chosen as the projection for the implantation. In case of difficulties or differences, corrections were made ac cording to the previous angiography.
When this was not feasible, positioning was performed using two pigtail or Amplatz AL2 catheters placed in the right and left sinuses, and then the angiographic projection showing cusp overlap was sought.
Usually, the COVL projection coincides with a caudally oriented right oblique projection.
The target was implantation approximately 2-3 mm below the aortic annulus with respect to the non-coronary sinus.
At the time of the final deployment, overstimulation with temporary pacemaker at 120 beats per minute was used to achieve system stability.
Both pre-dilation with a balloon diameter lower than that of the aortic annulus and post-dilation were performed according to the operator´s criterion.
All the patients were followed-up at 30 days with a face-to-face visit and analysis of PVR by Dopper-echocardiography performed 30 days after the implant.
Ethical considerations
The study was conducted following the recommendations of the Declaration of Helsinki and the International Conference of Good Clinical Practice. All patients signed an in formed consent form before participating in the study. Their identity was preserved for the moment of the analysis.
Statistical analysis
Continuous variables are presented as mean and standard deviation and categorical variables as absolute value and percentage. The Student’s t test was used to compare continuous variables and the chi-square test or Fisher's exact test was used for categorical variables. A p value < 0.05 was considered statistically significant.
RESULTS
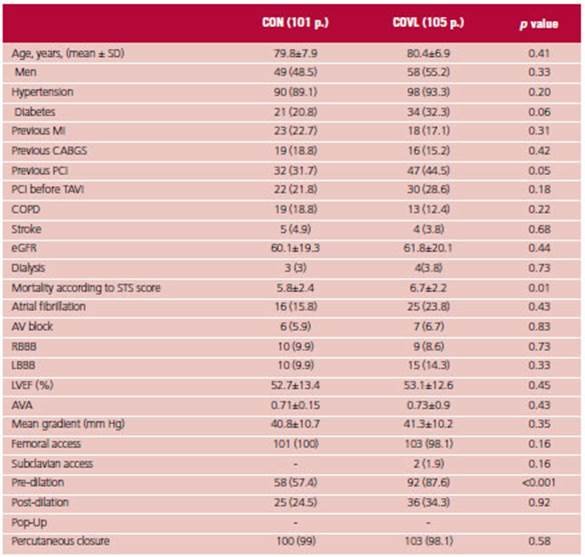
The clinical characteristics of the populations were similar; age was 79.8 ± 7.9 years vs. 80.4 ± 6.9 years in the CON strategy vs. COVL strategy, and 48.5% were male vs. 55%. There was no difference in the prevalence of hypertension, previous MI, history of coronary artery surgery, percutaneous coronary intervention (PCI) before TAVI, chronic obstructive pulmonary disease (COPD), renal function, and dialysis. Diabetes and PCI were slightly more common in the COVL group (20.8% vs. 32.3%, p = 0.06, and 31.7% vs. 44.8% p = 0.05, respectively).
The STS risk score was greater in the COVL group (5.8 ± 2.4 vs. 6.9 ± 2.2, p = 0.01).
There were no differences in the baseline ECG in terms of previous conduction disorders as atrioventricular (AV) block, right bundle branch block (RBBB), LBBB, or atrial fibrillation.
There was also no difference in left ventricular ejection fraction, mean trans-aortic gradient or aortic valve area.
Femoral access was used in all the p except for 2 p. in the COVL group who underwent subclavian access. Predilation was greater in this group (87.6% vs. 57.4%, p<0.001) with no differences in the use of post-dilation. There was no difference in the use of percutaneous closure, which was done with any of the following devices: PROSTAR XL® (ABBOTT Vascular, Santa Clara, California) and Proglide® (ABBOTT Vascular, Santa Clara, California). (Table 1).
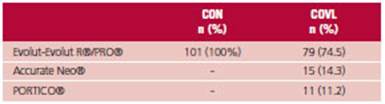
Self-expanding valves were implanted in all the patients. (Table 2)
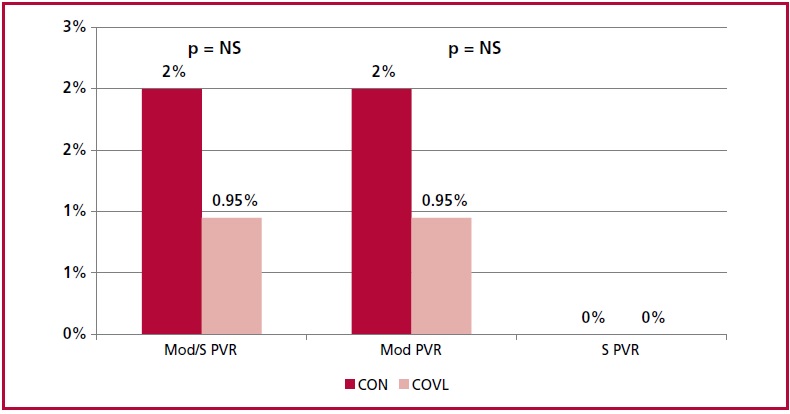
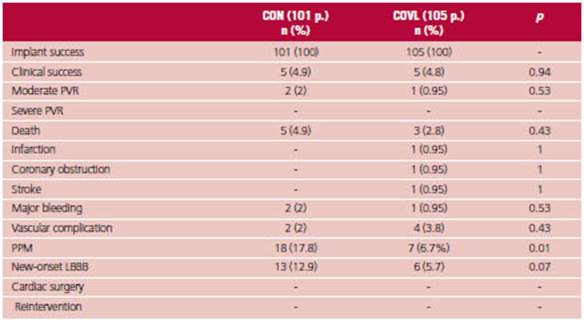
There was no difference in the PEP at 30 days be tween both strategies (Figure 1), with 2% incidence of moderate PVR in the CON group and 0.9% in the COVL group, and there were no cases of severe PVR. There was no difference in mortality, acute MI, coronary obstruction, stroke, cardiac surgery, reoperation, vascular complication or major bleeding.
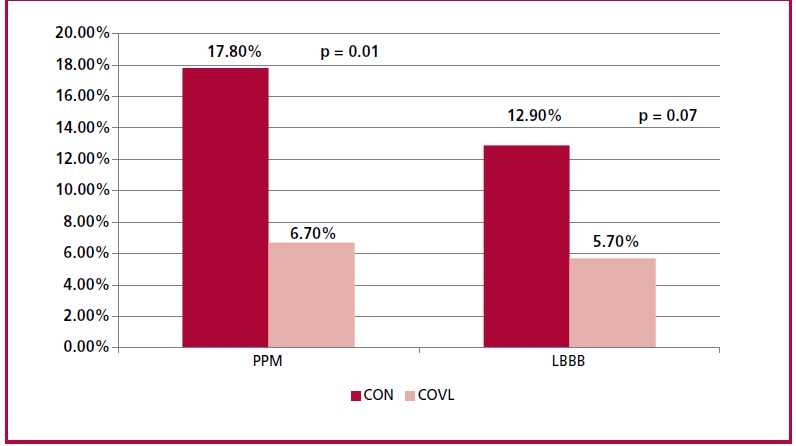
The COVL strategy resulted in a significant reduction in conduction disorders after implantation, with a lower need for PPM (6.7% vs. 17.8%; p = 0.01) and a trend towards less development of new LBBB (5.7% vs. 12.9%; p = 0.07). Of the patients requiring PPM, 6 were ≥ 80 years and only one patient presented normal sinus rhythm. One patient had previous atrial fibrillation, one presented first degree AV block, an other had LBBB, and one had trifascicular block. (Table 3) (Figure 2)
DISCUSSION
In our series, TAVI with the COVL technique was not significantly associated with a difference in the pres ence of PVR compared with the conventional strategy, but there was less need for PPM and lower incidence of LBBB, with no differences in mortality, acute MI, stroke, coronary obstruction, major vascular complications and major bleeding, cardiac surgery or reintervention.
Of the 7 p. in our series requiring PPM, 6 were ≥ 80 years and 5 presented a significant conduction disorder.
The presence of PVR has been related to valvular calcification with a generally asymmetrical pattern with dominating non-coronary leaflet and sinus calcification in most p. 15 Different studies have analyzed the presence of calcium and its quantity in the implant site 16,17 and demonstrated that asymmetry in calcification is related to PVR. 18 Some authors analyzed the presence of calcium in the left ventricular outflow tract which could be a risk factor for PVR. However, we must also consider that calcification of the annulus may contribute to annular rupture, especially in balloon-expandable valves in case of aggressive pre-dilation or post-dilation and need for a second valve. 19-21
Total device landing zone calcium volume could predict the degree of PVR: none with 389 mm3, mild with 371 mm3, moderate with 690 mm3 and severe PVR with 777 mm3. We should also analyze asymmetry in the CT scan as it is a predictor of PVR. 22
The risk of moderate or severe PVR is lower in women; this seems to be related to a smaller diameter annulus that facilitates perivalvular sealing. 23
The presence of moderate to severe regurgitation was more common at the beginning of the experience and with the first-generation devices. The learning curve of the operators, which has improved the implants, and the new devices with a perivalvular "skirt" at the base to improve sealing, have led to a significant reduction of this phenomenon.
Although the use of the COVL technique seems to help in the same sense (in fact, in our series PVR de creased by 50%), a greater number of patients would be needed to obtain considerable higher differences than those of our experience.
The presence of moderate or severe PVR is often associated with low functional class dyspnea or even hemolysis. One of the treatments proposed to avoid surgery is closure of PVR with plugs under transesophageal Doppler echocardiography. Although this strategy is not easy to perform and is not frequently used, it has demonstrated favorable outcome in some publications with a low rate of complications. 25
An important meta-analysis evaluated the outcome of patients with PVR at 4 years. Those with moderate to severe PVR had higher mortality than those with mild or minimal PVR. But when first-generation valves were compared with second-generation valves, the incidence of PVR was significantly lower with second-generation valves. Mild PVR had higher 4-year mortality rate than minimal PVR. For this reason, it is currently necessary to be extremely accurate at the time of implantation, choosing the most suitable device and performing post-dilations if necessary to leave the minimal regurgitation possible; ideally, absolutely no regurgitation.
Our group started several years ago with the implantation of self-expandable valves with the COVL strategy. In our publications, we demonstrated a significant decrease in the need for PPM after implantation and a trend towards lower LBBB without increasing the incidence of complications or valve embolization. In our publications, implants were per formed with different self-expanding valves in the COVL group. 11,12
We believe that this strategy offers benefits, and although we did not observe a significant difference in the incidence and severity of PVR that would have an impact on survival, the reduction in conduction disorders after implantation justifies its implementation, since conduction disorders also have an impact on survival and on hospital and long-term costs. 26,27
Study limitations
The lack of randomization and the fact that this analysis was performed in a single center are the limitations of this study. In order to try to avoid the influence of the learning curve, a historical series of the last 101 patients treated with the CON strategy was compared with the first 105 patients treated with the higher transcatheter aortic valve implantation strategy.
CONCLUSIONS
In this single-center series, the strategy of high transcatheter aortic valve implantation showed no difference in the presence of moderate or severe PVR compared with the conventional strategy with no differences in complications and was associated with a lower need for definitive pacemaker and a trend towards lower incidence of LBBB at 30 days.