INTRODUCTION
According to recent WHO reports, 40% of the world’s adult population consumes alcohol. In Europe this value is even higher and reaches 60%. To alcohol related diseases belong cardiac arrhythmias, especially atrial fibrillation, alcohol cardiomyopathy, hypertension, stroke, epilepsy, depression, hepatic steatosis, and cirrhosis, as well as pancreatitis and numerous cancers. Alcohol related myocardial damage in early stages is believed to be reversible after cessation of drinking, but the predictors of reversibility are not well-defined. Similarly, the exact alcohol amount that can be recommended, while still remaining safe for health, changes according to different subjects, their sex, age, and general as well as cardiovascular status. Nevertheless, the knowledge in this field has been recently intensively widened with studies providing associations between clinical and laboratory data, consumed amount of alcohol, and novel echocardio graphic parameters describing heart morphology and function. The present review shortly recalls the epidemiology and pathophysiology of alcohol overuse, as well as summarizes the current data concerning the impact of alcohol consumption on the cardiovascular system and heart function.
Epidemiology of alcohol consumption
According to the World Health Organization (WHO) report from 2018, approximately 2.4 billion people in the world drink alcohol, 43% of the world’s adult population over 15 years old. 1 The highest alcohol consumption burden is reported in Europe and reaches 60% of the population. The second region with highest alcohol consumption is South and North America, in which it achieves 54%. In comparison, tobacco use is estimated in 1.33 billion persons (22%) in a world-wide scale. 2
The world’s alcohol consumption, presented as lit ers of pure ethanol per capita per year (APC - alcohol per capita, including persons older then >15 years), has been increasing since 2000. According to the cited WHO report, in 2000 APC reached 5.7 liters and it rose to 6.4 liters in 2016. Interestingly, simultaneously with the increase of the APC index, the number of alcohol drinkers decreased by about 683 million. 1
As far as the sex-difference in consumption is concerned, in Europe 69% of men and 50% of women drink alcohol, whereas in South and North America alcohol is consumed by 67% of men and 42% of women. 1
The alcohol impact on health
Genetic factors may have an influence on the level of alcohol consumption as well as on ethanol tolerance. Holmes et al. investigated a group of 260 000 subjects to evaluate the impact of the rs 1229984 mutation in the gene encoding 1B alcohol dehydrogenase (ADH1B) on drinking behavior. The rs 1229984 mutation seems to be related to specific drinking behavior characterized by the tendency to consume lower alcohol volumes (17% lower compared with the group without this mutation). Since the mutation carriers were exposed to more severe symptoms connected with alcohol metabolism, by limiting the amount of consumed ethanol, they showed thereafter lower risk of alcohol related complications. In this study, the lower risk of ischemic heart disease was observed in the group with the mentioned mutation (Odds Ratio [OR] 0.90 and 95% Confidence Interval [95%CI] 0.84- 0.96). 3
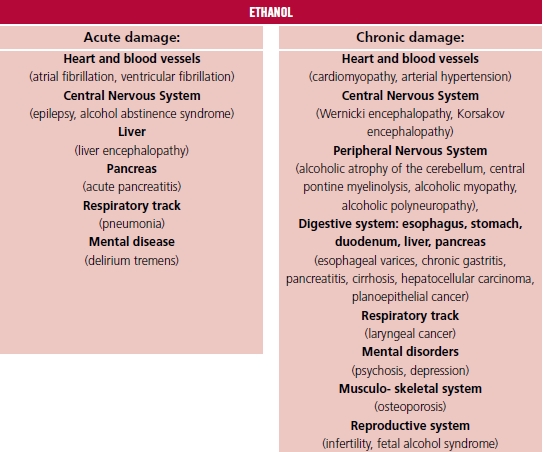
Nevertheless, the beneficial effect of low to moderate alcohol consumption on the human body was also postulated. The beneficial effect was jointed with documented reduction of the onset of ischemic heart disease, stroke, and diabetes. 1 In a meta-analysis performer by Di Castelnuovo and co-authors, based on 209 418 patients, cardiovascular risk reduction was observed (relative ratio [RR] 0.68, 95%CI 0.59 - 0.77) in the group consuming 150 mL wine per day, compared with teetotalers. 4 The beneficial effect of wine and beer was connected with the presence of specific ingredients belonging to the polyphenols, as resveratrol, whose molecular effect is based on triggering signal tracks like Nrf2 (nuclear factor erythroid 2-related factor 2), NF-κB (nuclear fac tor kappa-light-chain-enhancer of activated B cells), Sirt1 (NAD-dependent deacetylase sirtuin-1), AMPK (5'AMP-activated protein kinase), as well as the reduction of oxidative stress and apoptosis, which was observed in experimental studies. 5,6 In a meta-analysis including 1 902 605 participants, low alcohol doses (10 - 14 grams per day) induced 18% reduction of diabetes onset in women. 7 The postulated mechanism of this effect was the higher insulin sensitivity related to alcohol consumption. The variety of harmful alcohol impacts on the human body is presented in Figure 1. 1,8,9
ETHANOL
Alcohol metabolism
In the main pathway of alcohol metabolism two en zymes are engaged: alcohol dehydrogenase (ADH) and P450 2E1 cytochrome (CYP450). The intermediate product of this reaction is acetaldehyde, respon sible for the toxic influence on the human body. Fur ther, acetaldehyde is metabolized by acetaldehyde dehydrogenase and acetaldehyde oxidase to acetic acid, which enters the Krebs cycle. A certain amount of the acetaldehyde leaves the liver and is directed to peripheral tissues where it is metabolized to acetyl coenzyme A (acetyl - CoA) as well as to cholesterol, steroids, fatty acids, ketones and CO2 (Figure 2). Al cohol metabolism starts in the gastric mucosa whose cells contain ADH. After absorption in the gastro intestinal system through the portal vein, alcohol goes to the hepatocytes where the main part of the metabolism is performed, since the hepatocytes’ cytoplasm, mitochondria and microsomes contain the highest amounts of ADH and CYP450. 10 A variety of factors can modify the rate of alcohol metabolism. The afternoon body temperature increase, connected with the daily cycle, accelerates alcohol metabolism. Physical exercise has insignificant influence on al cohol metabolism acceleration, which is connected rather with body temperature elevation. Young age is a factor reducing the pace of alcohol metabolism due to lower expression of ADH and CYP450. The ADH Beta3 isoform characterizes higher efficiency of alcohol metabolism compared with other isoforms. Cederbaum and co-authors observed the higher prevalence of ADH Beta3 isoform among Afro-Americans (15%), and less frequent among Japanese, South Americans (except indigenous peoples) and Europeans. The ADH Beta3 isoform prevalence in this groups determines the rate of alcohol metabolism and the risk of alcohol related complications. 10 According to lower content of body water compared with men, women are more sensitive to alcohol toxic effects. Other factors having impact on alcohol metabolism are nutrition level, and exposition on other drugs like nicotine, cannabinoids, and cocaine. 11,12
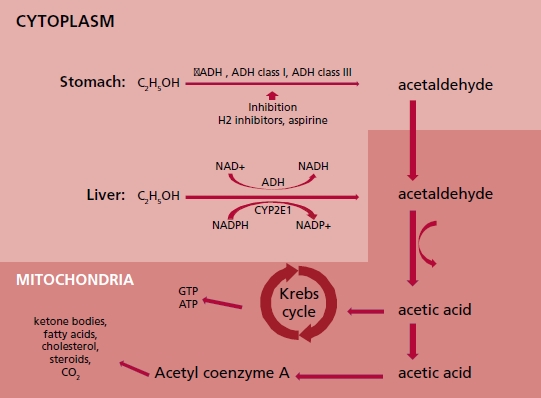
Fig. 2 Diagram illustrating alcohol metabolism in stomach and liver cells. σADH: alcohol dehydrogenase sigma iso form. ADH class I: alcohol dehydrogenase class one.ADH class III: alcohol dehydrogenase class three. ATP: adenosine triphosphate. CYP2E1: Cytochrome P450 2E1. ALDH2: mitochondrial aldehyde dehydrogenase 2/ acetaldehyde oxidase. C2H5OH: ethanol. CO2: carbon dioxide. GTP: Guanosine triphosphate. H2: histamine receptor type 2. NAD+: oxidized nicotin amide adenine dinucleotide. NADP+: oxidized nicotin amide adenine dinucleotide phosphate. NADH: reduced nicotinamide adenine di nucleotide. NADPH: reduced nicotinamide adenine dinucleotide phosphate
Alcohol consumption influence on the cardiovascular system
First studies
In 1861 Friedrich L. Goltz documented the relation ship between heart hypertrophy and persistent alco hol overuse. Further, in 1873 Walter H. Walshe as the author of the medical textbook entitled “The Physical Diagnosis of Diseases of the Lungs” implemented the term “cirrhosis of the heart” to describe phenomena of fibrosis observed simultaneously in both the heart and the liver in patients with alcohol overuse his tory. 13,14 In 1884, in Munich, Otto von Bollinger described “beer heart” cases (“Bierherz”) and linked this observation to a history of beer overuse, reaching up to 432 liters of beer per year. The “beer heart” in histopathology studies is characterized by hypotrophy, fibrosis, and steatosis. 15,16 At the end of the 19th century Graham Steell proved the relationship between heart failure and chronic high-dose alcohol consumption. 17 In 1969, in Quebec, a series of patients with cardiomyopathy related to beer drinking were observed. All mentioned cases were reported in a seven-month period. Interestingly, all patients consumed beer derived from the same origin, and ingredient analysis proved excess cobalt concentration (in this time, cobalt was applied as foam stabilizer in beer). 18 The authors postulated alcohol and cobalt as the etiology of cardiomyopathy in the above-men tioned cases.
Cardiotoxic alcohol dose
One of the first reports mentioning the relationship between alcohol dose and its cardiotoxic impact was published by Tadashi Koide and co-authors in 1974. The authors investigated the association between left ventricular enlargement in chest radiograms and the dose of consumed alcohol. The study showed that a pure ethanol dose ≥125 mL per day was related to the increase of the cardio-thoracic index (CTI) above 0.5. Higher CTI rates were observed in 33% of patients drinking ≥125 mL per day and in 4% of patients drinking from 75 to 125 mL, as well as in 2.9 % of patients drinking <75 mL. The authors did not mention time duration of consumption in this group of patients. 19 Other authors suggest that J or U - shaped curves can describe the relationship between the alcohol dose and the harmful cardiac effect. 20 For example, Ronksley et al., in a meta-analysis including 84 studies, revealed that drinking 15 - 30 grams of pure alcohol per day resulted in the decrease of cardiovascular mortality rate, with hazard ratio (HR) 0.66 (95%CI 0.59 - 0.75), and in the group drinking 2.5- 14.9 grams per day, with HR 0.80 (95%CI 0.74 - 0.87). The decline in all-cause mortality was observed in the drinking group compared with teetotalers, with HR 0.87 (95%CI 0.83 - 0.92). In this study the negative al cohol impact (ascending J curve arm) was observed in groups drinking 30 - 60 grams per day (HR 1.15 [95% CI 0.98 - 1.35]). 21
In the studies considering chronic alcohol consumption and its relationship with cardiac toxicity, the alcohol dose is usually expressed as the amount of alcohol and the time duration of alcoholism. In the literature, the authors postulate a dose of 80 grams of pure ethanol drank every day during 5 years as a toxic dose. 22-25 Other proposed cut-offs are 40 grams per day, or on average of 14 grams per day during a minimum of 10 years. 20,26
Alcohol-induced hypertension
The relationship between alcohol consumption and blood pressure elevation can be temporary, and stop after alcohol drinking cessation. 27 The hypertensive alcohol effect seems to be independent of body mass index (BMI), tobacco use, physical activity and coexisting arterial hypertension history. 20 This effect is caused by a few mechanisms: the increased activity of the renin-angiotensin-aldosterone axis and sympathetic nervous system, increased cortisol secretion, changed insulin sensitivity, endothelial dysfunc tion and reduced nitric oxide production. 28 The modulation of the central nervous system activity after alcohol exposure is an important reason for blood pressure elevation. The alcohol vulnerable nervous tracks are the rostral ventrolateral medulla and in termediolateral nucleus, as well as the baroreceptor reflex, which passes through the nucleus solitarius, and causes a hypertensive reaction. 20 According to the European Cardiac Society guidelines for hypertension management, an alcohol dose above 14 alcohol units (AU) per week (one AU contains 10 grams of ethanol) for men and 8 AU per week for women is not recommended. 29 The rise of the consumed dose has a linear positive correlation with the risk of arterial hypertension development. 28 This relationship is sex independent, but in women can be observed from a dose of 2 AU per week. (30) In a meta-analysis performed by Roerecke and co-authors, mean reductions of 5 mmHg in systolic blood pressure and 4 mmHg in diastolic blood pressure were observed in patients who reduced the consumed dose from 6 AU per day to 0 AU. 31
Dilated cardiomyopathy
The prevalence of dilated cardiomyopathy in the study performed by Fernández-Solá and co-authors was higher among patients consuming alcohol compared with the general population and reached 0.43% in males (mean pure alcohol amount consumed during lifetime 30 ± 7 kg/kg body mass during an average time of 29 ± 6 years), and 0.25% among women (mean pure alcohol amount consumed during lifetime 17 ± 7 kg/kg body mass during an average time of 23 ± 7 years), whereas in the general population dilated cardiomyopathy prevalence reaches 1:2500 individu als (0,4‰). 32-34 In the western countries, alcohol overuse is the leading cause of non-ischemic cardiomyopathy. In the literature, the estimated rate of alcohol overuse among patients diagnosed with dilated cardiomyopathy ranges from 3.8% to 47%. 23,35 Factors as malnutrition, kwashiorkor, vitamins, electrolytes and microelement deficiencies (sodium, potassium, calcium, magnesium, phosphorus and selenium), as well as the exposition to other psychostimulants like cocaine, amphetamine and nicotine, increase the risk of alcoholic cardiomyopathy. 33,36
There are no typical histopathological signs of alcoholic cardiomyopathy. Similar to other dilated cardiomyopathy etiologies, myofibril atrophy, mitochondriopathy (the abnormal differentiation of the mitochondria in size and forms due to the chronic exposition to toxins including ethanol, and the presence of megamitochondria), cardiomyocyte necrosis and fibrosis are present. 11,14 Myocardial muscle exposition to alcohol in the extracellular matrix leads to the activation of fibroblasts and the excessive production of type I collagen. 37 Additionally, in fibroblasts exposed to alcohol, the pro-inflammatory signal tracks are activated (the MAPK protein kinase groups, the transcription factor STAT3 and the nuclear factor NF- κB) causing the pro-inflammatory cytokine release from the fibroblast (IL-6, TNF-α, IL-1β, IL-33), and cardiomyocyte dysfunction. 37,38 This process leads also to lower expression of genes encoding contractile proteins, like Acta1, Actc1 (encoding actin) and Myh7 (encoding the myosin heavy chain alpha and myosin beta). Moreover, alcohol exerts also a direct influence on cardiomyocytes (mediated by TNF-α, without fi broblast as a mediator of the reaction), intensifying the processes leading to a decrease of muscle fiber contractility. 37
Acetaldehyde is an alcohol metabolite. Like etha nol, acetaldehyde has a negative effect on the heart muscle, but the influence of its cardiotoxic properties is even stronger than that of alcohol. 33 Both molecules have direct impact on protein synthesis in the myocytes and lipid peroxidation, reduce the contractility of myofibrils, and increase oxidative stress. 33,39 In magnetic resonance imaging, Liu S et al. observed shorter native T1 values and greater extracellular volume in the myocardium (similarly to images observed in heart amyloidosis and sarcoidosis) of a group of patients consuming >28 grams of ethanol per day, during a minimum of 10 years, compared with a control group (including persons drinking <100 grams per month). Similar changes in magnetic resonance imaging are observed when the content of fat molecules in the cell is increased. 26 Matyas and al. observed heart steatosis in the mice exposed to ethanol compared with the control group receiving a calorie-adjusted diet but without ethanol exposure. 40
In images recorded by positron emission tomography with carbon 11-labeled acetate, the decreased metabolic activity of the myocardium has been observed in chronic alcohol overusers (26 years on aver age) consuming high alcohol doses (167 grams of pure ethanol per day) compared with patients drinking for a similar period a lower amount of alcohol (50 grams per day), which suggests a decrease in mitochondrial function after chronic alcohol exposure. 26
The threshold of myocardial vulnerability to al cohol varies from person to person and has not been clearly defined. The literature suggests that the sensitivity can be also determined by genes. It is estimated that the genetic background of susceptibility is present in 20% to 37% of dilated cardiomyopathy cases. Currently, the possible mutations related to alcohol cardiomyopathy occurrence are identified in over 50 genes, 41 the most common encoding proteins being titin, posterophyllin - 2, myosin - binding protein C, desmoplakin, ryanodine - 2 receptor, desmokolin - 2, desmoglein- 2 and SCN5A. 42 Ware and co-authors assessed the effect of the presence of genes related to the occurrence of dilated cardiomyopathy on cardiac function in patients consuming alcohol. The group consisted of 715 patients aged 55 ± 14 years and in cluded 70% men. In the subgroup with the titin muta tion (titin is the largest human protein, crucial for the molecular basis of diastolic function), drinking excessive amounts of alcohol (>21 AU/week in men or >14 AU/week in women) resulted in an 8.7% reduction of left ventricular ejection fraction, LVEF (95%CI 2.3% - 15.1%; p =0.007), compared with patients without this mutation. 43 The titin-encoding gene variants associated with the development of dilated cardio myopathy were more prevalent in the alcoholic cardiomyopathy group compared with the control group. In the group of patients with alcoholic cardiomyopathy, a relationship between the amount of alcohol consumption and a decrease in LVEF was observed. This means that in sensitive individuals, alcohol can also be a trigger in the dilated cardiomyopathy development process. In addition, the finding of a greater prevalence of titin gene mutations in the group with alcoholic cardiomyopathy indicates the need for the active search of cardiomyopathy signs and symptoms among the relatives of patients with this diagnosis. 44
The toxic effect of alcohol metabolites on the heart results in left and right ventricular muscle remod eling, atrium enlargement, and secondary mitral and tricuspid valve regurgitation. 11 Based on the literature, it is postulated that the diastolic dysfunction occurs first, followed by left ventricular wall hypertrophy comparable to that observed in arterial hypertension. Further, left ventricular systolic diameter (LVSD) and the left ventricular diastolic diameter (LVDD) increase is observed, which parallels LVEF reduction. 11,25,33,45,46
Current available data suggest that chronic alcohol exposure also damages the right ventricle. 47 In a study of the cardiac effects of a single exposure to alcohol, Cameli and co-authors observed a group of 64 volunteers (mean age 25 ± 4 years, 29 women) before and 60 minutes after 0.5 gram per kg alcohol consumption, and compared it with a control group. TAPSE (tricuspid annulus plane excursion) was 22.1 ± 3.3 mm in the studied group vs. 24.0 ± 3.1 mm in controls, p = 0.003; and pulmonary artery systolic pressure (PASP) amounted to 23.7 ± 3.2 mmHg in the exposed group vs. 20.2 ± 4.9 mmHg in controls, p = 0.0002. 48
This influence of alcohol on right ventricular hemodynamic function can be related to increased pulmonary artery resistance due to vasoconstriction caused by leukotrienes released from leukocytes after alcohol exposure. 49
Guzzo-Merello and co-authors postulated that the lack of β-blocker treatment, the occurrence of atrial fibrillation and the widening of the QRS complex are independent predictors of worse prognosis in alcoholic cardiomyopathy, with an increased incidence of cardiac death and heart transplantation. In patients who reduced their alcohol intake below 80 grams per day, the 59 - month risk of cardiac death or heart trans plantation was reduced to a level comparable with that of the general population. 23 The lack of abstinence in patients with alcohol-related dilated cardiomyopathy resulted in very high mortality, reaching 50% within 4 years, which poses alcoholic cardiomyopathy near to patients with cancer diagnosis. 50 The pleiotropic effects of alcohol on the heart are schematically shown in Figure 3.
Since the key issue related to early diagnosis of alcohol related cardiomyopathy may be the usage of adequate diagnostic methods, it is worth mentioning the still underused potential of the adapted protocols of stress echocardiography in the early detection of dias tolic dysfunction as well as pulmonary hypertension. 51 Vriz and co-authors performed exercise Doppler echocardiography in a group of 155 hypertensive patients and in 145 healthy subjects, and documented that TAPSE during exercise was lower in the hypertensive group, whereas PASP as well as its value in dexed to the cardiac output achieved during exercise was higher. 52
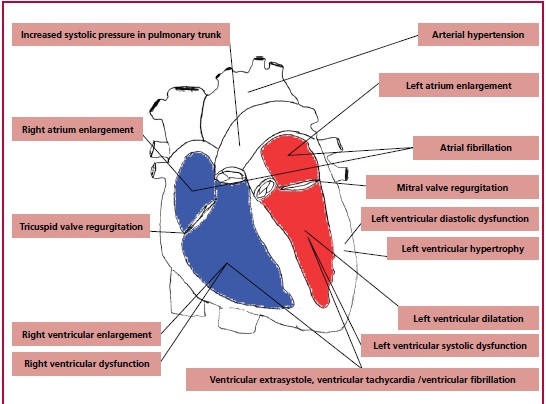
Fig. 3 D-dimer boxplot between patients undergoing computed tomography pulmonary angiography diagnosed with (1) or without (0) pulmonary embolism. a) pre- COVID-19 phase, b) COVID-19 phase
In a recently published analysis of our group, the median and interquartile range (IQR) of left ventricular mass index was 119 (91-155) g/m² in patients drinking alcohol (median 30 (12-51) AU per week) vs. 93 (75-110) g/m² in a control group (drinking up to 2 AU per week), p = 0.008; and the relative wall thickness in the alcohol group was 0.5 (0.4-0.6) vs. 0.4 (0.4-0.4) in controls, p = 0.001. In the same study, global longitudinal strain (GLS) and layer strains showed lower absolute values in alcohol overusers as compared to controls. All abnormal strain parameters were associated with a more frequent composite end point occurrence (higher clinical risk of death or cardiovascular hospitalization). The best absolute cut-off values for outcome prediction were: GLS <18%, layer endocardial strain <19%, and layer epicardial strain <15%. 53
Alcohol related arrhythmias
Atrial fibrillation (AF) is the most common arrhyth mia observed among alcohol abusers. Larsson and al. proved that during chronic alcohol exposure, an in crease in consumption of one AU per day increases the risk of AF by 8%. 54 Most often, arrhythmias are observed as a consequence of a single high alco hol dose intake in a short time and numerous authors have mentioned this as “holiday heart syndrome”. This syndrome was first reported in 1978 by Ettinger et al. 55,56 Chronic alcohol consumption is positively associated with increasing risk of AF in the long-term follow-up. In the study performed by Larson and al. (12 years of follow-up), AF RR (95%CI) for 7-14 AU/ week was 1.12 (1.02 - 1.23), and for >21 AU/week, 1.43 (1.25 - 1.65) compared with controls. 54 Arrhythmias are more often observed in patients diagnosed with cardiomyopathy than in those without structural abnormalities. Moreover, episodes of abstinence may be triggers of arrhythmia in chronically alcohol overusing patients, due to the increased adrenergic activity observed at the beginning of abstinence and the coexisting deficiencies of macro and micronutrients (sodium, potassium, magnesium, calcium, phosphorus, selenium) and vitamins (thiamine) associated with previous alcohol abuse. 33,57,58
Cirrhotic Cardiomyopathy
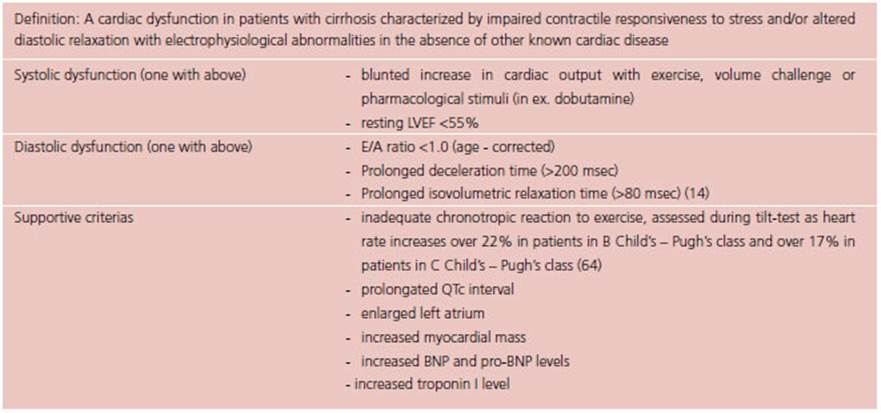
The disorders observed in the hepato-cardiac syn drome may also contribute to the pathogenesis of alcoholic cardiomyopathy. 59 Systolic function is decreased both at rest and during stress, the impairment associated with a decrease in the activity of β1- receptors through a reduction in G protein expression in the cytosol, related to liver impairment. 59-61 In parallel, diastolic dysfunction is observed, associ ated with left ventricular hypertrophy, fibrosis, and endothelial dysfunction. 59,62 These factors lead to secondary higher heart rate and increased cardiac output, which result in a hyperkinetic cardiomyopathy. Diagnostic criteria of Cirrhotic Cardiomyopathy have been established in 2005 during the World Congress of Gastroenterology and are displayed in Table 1. 59,63
Summary
The recent data show that chronic overuse of alcohol may lead to cardiovascular dysfunction, starting from traditionally judged as low ethanol doses, since the burden of arrhythmias, including atrial fibrillation, increases even in moderate alcohol consumers.
The other common mechanisms of the disadvanta geous impact of ethanol are related to the development of hypertension and its direct aftermath, hypertrophy, fibrosis, and diastolic dysfunction.
Since the chance of the reversibility of cardiac remodeling depends on the early diagnosis of cardiac dysfunction, the wider application of novel and sensitive methods of myocardial function assessment, including longitudinal strain of the left and right ventricles, as well as the adapted protocols for stress echocardiography, should be recommended.