Radiofrequency catheter ablation with pulmonary vein isolation for treatment of atrial fibrillation (AF) is a minimally invasive procedure with low rate of complications. One complication is pulmonary vein stenosis, with an incidence between 0.3 and 3.4%, but associated with high morbidity. 1-3 Symptoms of pulmonary vein stenosis include dyspnea, cough, hemoptysis, and chest pain. 4 We report a case of hemoptysis secondary to pulmonary vein stenosis after radiofrequency catheter ablation, treated with angioplasty and stent implantation.
A 42-year-old male patient with a history of radiofrequency catheter ablation of the pulmonary veins, performed in another center, and community-acquired pneumonia which was hard to treat despite the use of different antibiotic regimes, presented to the emergency department of our institution 5 months after the procedure with intermittent hemoptysis and pain in the left hemithorax.
On admission, the patient was hemodynamically stable, without fever and with no signs of heart failure. The laboratory tests showed: hematocrit 37%; white blood cell count 5490/mm3, 229 300 platelets/mm3, erythrocyte sedimentation rate 25 mm/h, and high-sensitivity C-reactive protein level 10.6 mg/L. As acute pulmonary embolism (PE) was suspected, the patient underwent chest computed angiotomography, which was negative for PE but showed areas of ground-glass opacification, with a tendency to consolidation, that were diffusely distributed in the left lung upper lobe.
The patient was clinically stable and was discharged with empiric treatment for suspected atypical pneumonia and was followed up in an outpatient basis. Because of the recent history of AF ablation and the pattern evidenced in the chest computed tomography, we decided to perform cardiac computed angiotomography to evaluate the pulmonary veins which had not been correctly visualized in the angiography requested to rule out PE. The scan showed stenosis of the left superior pulmonary vein (LSPV), with a diameter of 8.4 mm × 17 mm in length, pronounced narrowing in flute beak appearance, and critical luminal reduction with a trajectory of about 16 mm until reaching the patent intraparenchymal portion (Figure 1A). The other pulmonary veins were patent with no signs of stenosis.
In the presence of a patient with hemoptysis and symptoms of persistent pneumonia and stenosis of the LSPV, we decided to perform angioplasty of the pulmonary vein with stent implantation. A transesophageal echocardiogram (TEE) was performed before the procedure, which demonstrated increased velocities (peak velocity 2.4 m/s, peak gradient 23 mm Hg) in the vein, confirming the diagnosis (Figure 1B). Transseptal puncture was carried out through a right femoral venous access and under TEE guidance. A selective venography confirmed LSPV stenosis (Figure 2A). Then, coronary balloon catheters with increasing diameters were advanced for pre-dilation. A conventional stent with high radial strength (Herculink 7.0 × 18 mm) was implanted, with adequate angiographic results (Figure 2B). Pressures and velocities decreased in TEE (peak velocity 1.3 m/s, maximum gradient 7 mm Hg).
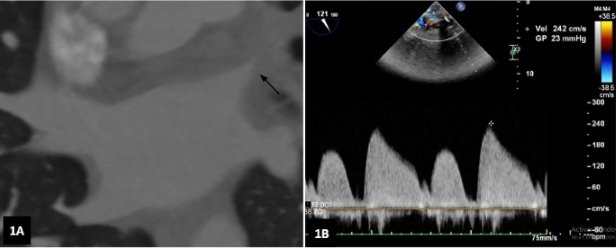
Fig. 1 A. Computed angiotomography showing stenosis of the left superior pulmonary vein (arrow). B. Left superior pulmonary vein velocity measured by transesophageal echocardiography.
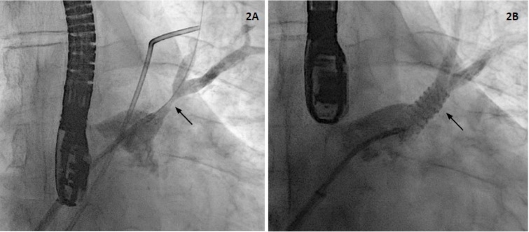
Fig. 2 A. Venography demonstrating stenosis of the left supe rior pulmonary vein (arrow). B. Venography after stent implantation (arrow) in the left superior pulmonary vein.
The patient was discharged 24 hours later, on anticoagulation with rivaroxaban 15 mg/d and antiplatelet therapy with clopidogrel 75 mg/d. At 3-month follow-up, dyspnea, cough and pain in the left side of the chest has disappeared.
Pulmonary vein stenosis after radiofrequency catheter ablation is a rare and underdiagnosed complication because symptoms can be mistaken for other conditions as pneumonia, asthma, and PE, among others. 4 The proper diagnosis of this condition is of utmost importance, as delayed diagnosis can lead to total occlusion of the pulmonary vein, resulting in pulmonary infarction and massive hemoptysis. Magnetic angioresonance and computed angiotomography are the most accurate diagnostic methods for detecting pulmonary vein stenosis. The severity of stenosis is defined by the percentage of lumen involved and is classified as mild (<50%), moderate (50%-70%) and severe (>70%). The development of symptoms is usually associated with severe stenosis or total occlusions, which can sometimes be asymptomatic. Transesophageal echocardiography is a very useful diagnostic method to guide the procedure, since it provides information on the structure of the left atrium, optimizes manipulation of materials as guide wires and balloon catheters during the procedure, and adds the evaluation of pulmonary veins flow and gradients for the diagnosis of stenosis and for evaluating treatment effectiveness. 5 In this case, we used the same procedure to provide diagnosis, guide transseptal puncture (which is ideally inferior and anterior for better orientation to the LSPV), measure velocities after the procedure, and rule out complications as pericardial effusion.
Treatment with angioplasty is intended to relieve venous pressure and improve perfusion of the affected lung. Compared to balloon angioplasty, stenting is associated with a lower rate of restenosis, especially when large stents (10 mm or more) are used. 5,6 Stent size is defined before the procedure by computed angiotomography and during the procedure by angiography, since the use of stents larger than the vein could lead to rupture and cardiac tamponade. Other treatments include lobectomy in patients with clinically significant pulmonary vein occlusion or stenosis in whom angioplasty has failed. 5
We report this case of hemoptysis, dyspnea and pain secondary to pulmonary vein stenosis that was successfully treated with angioplasty and stent implantation. The recognition of this condition, its timely diagnosis and adequate treatment is of utmost importance to reduce patient morbidity.














