Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cardiología
versão On-line ISSN 1850-3748
Rev. argent. cardiol. vol.92 no.1 Ciudad Autónoma de Buenos Aires mar. 2024 Epub 28-Fev-2024
http://dx.doi.org/10.7775/rac.es.v92.i1.20724
ORIGINAL ARTICLE
Left Ventricular Remodeling After Myocardial Infarction: A Perspective from Gated-SPECT Myocardial Perfusion Imaging
1 Cardiovascular Imaging Cross-Cutting Specialty. TCba, Buenos Aires, Argentina
Background:
Gated single-photon emission computed tomography (gated-SPECT) myocardial perfusion imaging is a suitable technique for measuring the infarct scar size and defining its territory. Analyzing patients with small and medium myocardial infarctions that develop reduced left ventricular ejection fraction (LVEF) could provide additional information of the factors that contribute to adverse remodeling and its outcome.
Objectives:
a) To evaluate the prevalence of reduced LVEF and associated factors in a population of patients referred for gated-SPECT imaging, and b) to define the prevalence of adverse remodeling and associated factors in the subgroup of patients with intermediate to low necrotic burden.
Methods:
We conducted a retrospective analysis of consecutive patients undergoing gated-SPECT imaging during 2017. Patients with significant valvular heart disease or arrhythmias that could difficult adequate ECG gating were excluded from the study. Adverse remodeling was considered as the combination of reduced LVEF (LVEF < 50%) with percent myocardium scar < 20%.
Results:
A total of 1902 patients were included. The prevalence of reduced LVEF was 8% (n = 148). On multivariate analysis, the variables with independent association with ventricular dysfunction were male sex (OR 2.50; 95% CI 1.30-4.90, p = 0.005), diabetes (OR 1.83; 95% CI 1.12-3, p = 0.01), and percent myocardium scar > 6.6 % (OR 39; 95% CI 25-61.28, p = 0.00001). In the subgroup of patients with scar burden < 20 % (n = 197), the prevalence of adverse remodeling was 25 % (n = 50). On multivariate analysis, diabetes (OR 2.83; 95% CI 1.31 - 6.1 p = 0.007) and male sex (OR 5; 95% CI 1.1 - 22.9, p = 0.007) showed an independent association with adverse remodeling.
Conclusion:
Gated-SPECT could be used to assess adverse remodeling and its associated factors. This assessment is the result of combining variables used in daily practice which do not require any additional software.
Key words: Gated-SPECT; Ventricular remodeling; Post-infarction ventricular dysfunction
INTRODUCTION
Adverse ventricular remodeling is a crucial factor in the development of heart failure after an acute coronary event. It is a process that involves changes in left ventricular (LV) geometry, volume, and function, and is a determining factor of cardiovascular morbidity and mortality. 1
Adverse LV remodeling is a maladaptive process of the heart to mechanical and neurohormonal changes in the regulation of ventricular size, shape, and function. Cardiomyocyte growth due to increased blood flow, such as during pregnancy, development, or exercise training, is considered physiological and reversible. On the contrary, adverse ventricular remodeling after an acute myocardial infarction (AMI) is associated with a high risk of heart failure and reduced survival. 2),(3
The goal of drug treatment is to improve the mechanics of contraction, for example by reducing the afterload with vasodilators, and to adjust the molecular mechanisms of remodeling. 4 Identifying predictors of remodeling in clinical, biochemical, and imaging data could reveal new targets for treatment. In this sense, understanding the variables that increase the risk of remodeling in patients with MI could represent an opportunity to adjust treatment and improve follow-up.
The infarct size has been described as a predictor of adverse remodeling in animal models. Gated- SPECT myocardial perfusion imaging is an appropriate method for measuring the infarct scar size and defining its territory. 5),(6 Analyzing patients with small and medium MIs that develop LV dysfunction could provide additional information about the outcome of adverse remodeling.
The aims of our study were: a) to evaluate the prevalence of reduced left ventricular ejection fraction (LVEF) and associated factors in a population of patients referred for gated-SPECT imaging, and b) to define the prevalence of adverse remodeling and associated factors in the subgroup of patients with intermediate to low necrotic burden.
METHODS
Experimental model Study population
We conducted a retrospective analysis of consecutive patients undergoing gated-SPECT imaging during 2017.
The imaging test was indicated by the treating physician due to one of the following reasons in patients with and without known coronary artery disease: 1) symptoms of angina or suspected coronary artery disease, 2) electrocardiogram (ECG) abnormalities suggestive of coronary artery disease, 3) abnormal exercise stress test, 4) intraventricular conduction disorders that required pharmacological stress test, 5) new onset symptoms with known coronary artery disease or recent myocardial infarction, 6) abnormal ventricular wall motion demonstrated by another imaging test. Patients > 18 years were included in the study.
Those patients with significant valvular heart disease or significant arrhythmias that could difficult adequate ECG gating were excluded from the study. The presence of coronary risk factors and clinical status were recorded.
Gated- SPECT protocol for image acquisition
A detailed medical record was obtained before the stress test. The Bruce protocol limited by symptoms was used in patients who underwent an exercise stress test. Heart rate and blood pressure were measured, and a 12-lead ECG was recorded at baseline, the beginning of each stage and during recovery. In patients undergoing pharmacological stress, dipyridamole was administered at 0.56 mg/kg intravenously over a 4-minute period and the radiotracer was injected 3 minutes after the completion of dipyridamole infusion, according to the recommendations of the American Society of Nuclear Cardiology (ASNC). 7
We performed a one-day protocol with injection of technetium- 99m methoxy isobutyl isonitrile (Tc-99m MIBI) at stress and rest using a dedicated gamma camera with dual-detectors and a 90° angle (Ventri camera system, GE Medical Systems, Milwaukee, Wisconsin, USA). Rest images were acquired 30 to 45 minutes after venous injection at rest, and stress images 15 to 20 minutes after peak exercise. The radiotracer dose used was 8-12 mCi in the first injection (rest or stress imaging) and 24-36 mCi in the second injection (stress or rest imaging) following the recommendations of the ASNC guidelines.
Image analysis and quantification
Data were acquired in a 128 × 128 matrix for 32 projections in a step and shoot format. SPECT analysis was performed according to the left ventricular 17-segment model of the American Heart Association. 8 For that purpose, reconstruction into long and short axis projections perpendicular to the heart axis was initially performed, followed by an automated quantitative analysis of the perfusion images using polar map format (normalized to 100%). Automated quantitative analysis was used as complementary data to semi-quantitative visual interpretation. The segments were scored on a scale of 0 to 4 through visual analysis of radiotracer uptake in each segment. The total sum of the scores in the stress and rest images provided the summed stress score (SSS) and the summed rest score (SRS), respectively. The summed difference score (SDS) was calculated as the difference between the SSS and SRS. A SRS greater than or equal to 2 was considered necrosis. The percent myocardium scar was calculated as SRS x 100/68 according to a previous publication. 9 End-diastolic volume (EDV), end-systolic volume (ESV) and LVEF were determined using QGS 2015.2 software program. Gated images were used to assess regional wall motion to discriminate between perfusion defects and attenuation artifacts. If soft tissue attenuation was suspected during the initial supine position acquisition, additional images were acquired in the prone position.
Definition of adverse remodeling
Reduced LVEF was considered as < 50% in rest images.
Adverse remodeling was considered as the combination of decreased LVEF with percent myocardium scar < 20%
Statistical analysis
Multivariate logistic regression was used to find associations. The dependent variables were reduced LVEF in the first analysis and adverse remodeling in the second. The following variables were considered in the search for an association: age, male sex, hypertension, dyslipidemia, current or former smoking, diabetes mellitus (DM), and percent myocardium scar. A cut-off point for myocardium scar significantly associated with ventricular dysfunction was established by a ROC curve and included in the model as a dichotomous variable. Continuous variables with normal distribution were expressed as mean standard deviation (SD) and were compared using the Student's t test, while those with non-gaussian distribution were expressed as median and interquartile range (IQR) and compared using the Mann-Whitney test. Categorical variables were expressed as proportions and were compared using the Fisher's exact test. A p value < 0.05 was considered statistically significant. All calculations were performed using Epi-Info 7.2.2.2 software package.
Ethical considerations
As this was an observational study, we only performed the tests requested by the treating physicians and analyzed the data obtained without any additional intervention. At our institution, patients sign an informed consent form in which they accept that data linked to their test results may be used anonymously for scientific purposes. The patients' identities were blinded during data analysis and preparation of the article.
RESULTS
A total of 1902 patients were included. Mean age was 63 ± 11 years; 12% of the patients had a history of AMI and 29% of myocardial revascularization (Table 1). The prevalence of reduced LVEF was 8% (n = 148). The ROC curve determined that a cut-off value of 6.6% of necrotic myocardium had the best accuracy to diagnose reduced LVEF, with an area under the curve (AUC) of 0.872, 95% CI 0.832-0.912 (Figure 1). On multivariate analysis, the variables with independent association with ventricular dysfunction were male sex (OR 2.50, 95%; CI 1.30-4.90, p = 0.005), DM (OR 1.83; 95% CI 1.12-3, p = 0.01), and percent myocardium scar > 6.6 % (OR 39; 95% CI 25-61.28, p = 0.00001) (Table 2).
Table 1 Baseline characteristics of the population.
| Variable | n = 1902 |
|---|---|
| Age, years, mean ± SD | 63 ± 11 |
| Male sex, % (n) | 66 (1263) |
| HTN, n (%) | 57 (1082) |
| DLP, n (%) | 49 (933) |
| DM, % (n) | 18 (350) |
| Smoking habits, % (n) | 9 (167) |
| MI, % (n) | 12 (235) |
| Myocardial revascularization, % (n) | 29 (544) |
| LVEF (%), mean ± SD | 65 ± 14 |
| Reduced LVEF, % (n) | 8 (148) |
| Necrosis on SPECT, % (n) | 16 (285) |
| Low necrotic burden, % (n) | 10 (197) |
DLP: dyslipidemia; DM: diabetes mellitus; HTN: hypertension; LVEF: left ventricular ejection fraction; MI: myocardial infarction; OR: odds ratio; PCI: percutaneous coronary intervention; SD: standard deviation; SPECT: single-photon emission computed tomography.
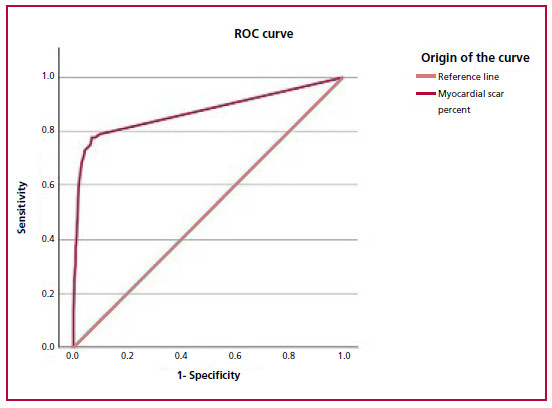
Fig. 1 ROC curve for estimating the myocardial scar percent threshold to induce ventricular dysfunction. The 6.6% cut-off point obtained the highest accuracy for LVEF reduction (sensitivity: 78%, specificity: 93%, AUC 0.872, 95% CI 0.832-0.912).
Table 2 Factors associated with reduced left ventricular ejection fraction.
| OR | 95% CI | p | |
|---|---|---|---|
| Age | 0.99 | 0.97 - 1.01 | 0.501 |
| Male sex | 2.50 | 1.30 - 4.90 | 0.005 |
| HTN | 0.87 | 0.50 - 1.30 | 0.501 |
| DLP | 1.30 | 0.84 - 20.00 | 0.204 |
| DM | 1.83 | 1.12 - 3 | 0.010 |
| Smoking habits | 0.53 | 0.22 - 1.26 | 0.204 |
| Myocardial scar percent > 6.6% | 39 | 25-61.28 | <0.001 |
CI: confidence interval; DLP: dyslipidemia; DM: diabetes mellitus; HTN: hypertension; OR: odds ratio
In the subgroup of patients with necrotic burden < 20 % (n = 197), the prevalence of adverse remodeling was 25 % (n = 50). On multivariate analysis, DM (OR 2.83; 95% CI 1.31 - 6.1 p = 0.007) and male sex (OR 5; 95% CI 1.1 - 22.9, p = 0.007) showed an independent association with adverse remodeling (Table 3).
Table 3 Factors associated with adverse remodeling
| OR | 95% CI | p | |
|---|---|---|---|
| Age | 1.01 | 0.98 - 1.05 | 0.202 |
| Male sex | 5.00 | 1.10 - 22.90 | 0.007 |
| HTN | 0.70 | 0.33 - 1.47 | 0.501 |
| DLP | 1.60 | 0.75-3.44 | 0.199 |
| DM | 2.83 | 1.31 - 6.10 | 0.007 |
| Smoking habits | 0.84 | 0.25 - 2.74 | 0.199 |
CI: confidence interval; DLP: dyslipidemia; DM: diabetes mellitus; HTN: hypertension; OR: odds ratio
DISCUSSION
The most significant findings of our study were that DM and male sex were independently associated with decreased LVEF. This association was also verified in the subgroup of patients with low scar burden. Patients with extensive myocardial necrosis are expected to have ventricular dysfunction. Therefore, we tried to elucidate any factors leading to ventricular dysfunction in patients with non-extensive necrosis (< 20%). The prevalence of this phenomenon, which we defined as adverse remodeling, was 25% (n = 50) in this group. In our cohort, some patients with infarctions of similar size developed adverse remodeling, while others maintained preserved LV volume and LVEF with little or no impairment. DM was found to be independently associated with this phenomenon (Figure 2). Our results are consistent with those reported by other publications. In patients with DM, the incidence of heart failure after a MI is 60-70% higher than in non-diabetics. 10
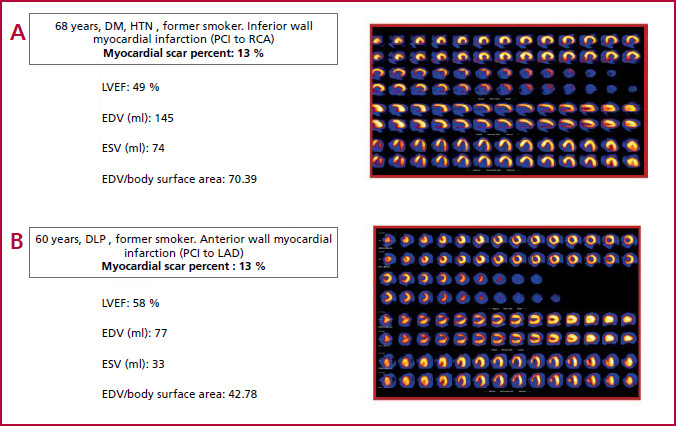
Fig. 2 a) Patient with diabetes and inferior wall myocardial infarction (myocardial scar percent: 13%), increased LV dimensions and ventricular dysfunction). b) Patient without diabetes and anterior wall myocardial infarction of similar size (myocardial scar percent : 13%), with normal LV dimensions and LVEF. DLP: dyslipidemia; DM: diabetes; EDV: end-diastolic volume; ESV: end-systolic volume; HTN: hypertension; LAD: left anterior descending artery; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; RCA: Right Coronary artery
In the 1980s, Jaffe et al. reported a higher incidence of congestive heart failure in diabetic patients, despite smaller infarct size. 11 Two decades later, Aguilar et al. described that patients with DM had a higher rate of cardiovascular events, including heart failure, than those without DM, despite having similar baseline characteristics such as infarct size and systolic function. 12 Prasad et al. reported that patients with diabetes were more likely to have abnormal myocardial perfusion than non-diabetics, despite similar TIMI 3 flow rates after primary percutaneous coronary intervention for an acute coronary syndrome. 13 Finally, in 2020, Van der Bijl et al. described a higher prevalence of diabetes in patients with adverse remodeling and an independent association between DM and heart failure hospitalizations in patients with acute coronary syndrome. 14
This suggests that there are other factors that contribute to myocardial dysfunction, which could explain the higher frequency of remodeling in this population. These factors include the activation of the inflammatory cascade, oxidative stress, microvascular obstruction, and diastolic dysfunction. 15
Cardiac magnetic resonance (CMR) imaging is currently the most commonly used non-invasive diagnostic modality for detecting remodeling, assessing its severity, and identifying associated factors. Compared with echocardiography, CMR presents better contrast resolution, higher reproducibility, and independence from acoustic windows, and may also provide valuable information regarding the presence and extent of myocardial fibrosis in the form of late gadolinium enhancement (LGE). The extent of LGE is directly related to reverse remodeling. 16 In this context, Monmeneu et al. reported that a lower prevalence of diabetes was related to a higher percentage of salvaged myocardium in patients with ST-segment elevation MI revascularized by angioplasty. 17 In a review that included 10 randomized studies, Stone et al. observed a strong graded relationship between infarct size, considered as every 5% increase, and mortality and heart failure hospitalization. In this study, the infarct size was measured by gated-SPECT in less than 30% of patients, while CMR was the most commonly used method to measure the necrotic burden. 18 While gated-SPECT is not commonly used in routine practice to assess remodeling, our study supports the hypothesis that it can be used for this purpose. There is limited literature analyzing ventricular remodeling after myocardial infarction using this imaging modality. Our group recently published that gated-SPECT could be used to noninvasively assess the main determinants of stroke volume, supporting the possibility of expanding the usefulness of this practice in evaluating patients with ventricular dysfunction. 19
Gated-SPECT is the most commonly used noninvasive test to rule out ischemia and evaluate risk in patients with a history of coronary artery disease. Strengthening its use in evaluating patients with ventricular dysfunction could simplify the assessment of individuals with ischemic cardiomyopathy. Additionally, the semi-quantitative visual score allows for easy quantification of necrosis without the need for additional software. This method is routinely used in clinical practice and has shown prognostic value. 20
Study limitations
The limitations of this study include its retrospective, single-center based and observational design, and the potential influence of unmeasured factors on ventricular remodeling. As we did not evaluate a gold standard for determining adverse remodeling, we cannot determine the accuracy of SPECT for assessing remodeling with our design. Additionally, we did not perform clinical follow-up to determine its impact on prognosis.
CONCLUSIONS
Gated-SPECT, the most commonly used noninvasive stress test for evaluating ischemic heart disease, could also be used to assess adverse remodeling and its associated factors. This assessment is the result of combining variables used in daily practice which do not require any additional software. This offers a window of opportunity to identify patients who may benefit from treatments currently recommended for more advanced stages. These hypotheses should be confirmed by future prospective studies with a larger number of patients and in randomized studies evaluating relevant clinical outcomes.
REFERENCES
1. Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370-80. https://doi.org/10.1056/NEJMra072139. [ Links ]
2. Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356-67. https://doi.org/10.1016/S0140-6736(06)68074-4. [ Links ]
3. Korup E, Dalsgaard D, Nyvad O, Jensen TM, Toft E, Berning J. Comparison of degrees of left ventricular dilation within three hours and up to six days after onset of first acute myocardial infarction. Am J Cardiol. 1997;80:449-53. https://doi.org/10.1016/s0002-9149(97)00393-7. [ Links ]
4. Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J Card Fail. 2022;28:e1-e167. https://doi.org/10.1016/j.cardfail.2022.02.010. [ Links ]
5. Hochman JS, Bulkley BH. Expansion of acute myocardial infarction: an experimental study. Circulation. 1982;65:1446-50. https://doi.org/10.1161/01.cir.65.7.1446. [ Links ]
6. Weisman HF, Bush DE, Mannisi JA, Bulkley BH. Global cardiac remodeling after acute myocardial infarction: a study in the rat model. J Am Coll Cardiol. 1985;5:1355-62. https://doi.org/10.1016/s0735-1097(85)80348-x. [ Links ]
7. Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606- 39. https://doi.org/10.1007/s12350-015-0387-x. [ Links ]
8. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. J Cardiovasc Magn Reson. 2002;4:203-10. https://doi.org/10.1081/JCMR-120003946. [ Links ]
9. Dorbala S, Ananthasubramaniam K, Armstrong IS, Chareonthaitawee P, DePuey EG, Einstein AJ, et al. Single Photon Emission Computed Tomography (SPECT) Myocardial Perfusion Imaging Guidelines: Instrumentation, Acquisition, Processing, and Interpretation. J Nucl Cardiol. 2018;25:1784-846. https://doi.org/10.1007/s12350-018-1283-y [ Links ]
10. Jen D, Stehlik J, Stan V, Kautzner J, Wohlfahrt P. Heart failure after myocardial infarction : incidence and predictors. Published online 2020. https://doi.org/10.1002/ehf2.13144. [ Links ]
11. Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J. 1984;108:31-7. https://doi.org/10.1016/0002-8703(84)90541-6. [ Links ]
12. Aguilar D, Solomon SD, Køber L, Rouleau JL, Skali H, Mc- Murray JJ, et al. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: the VALsartan In Acute myocardial iNfarcTion (VALIANT) trial. Circulation. 2004;110:1572-8. https://doi.org/10.1161/01.CIR.0000142047.28024.F2 [ Links ]
13. Prasad A, Stone GW, Stuckey TD, Costantini CO, Zimetbaum PJ, McLaughlin M, et al. Impact of diabetes mellitus on myocardial perfusion after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol. 2005;45:508-14. https://doi.org/10.1016/j.jacc.2004.10.054. [ Links ]
14. van der Bijl P, Abou R, Goedemans L, Gersh BJ, Holmes DR Jr, Ajmone Marsan N, et al. Left Ventricular Post-Infarct Remodeling: Implications for Systolic Function Improvement and Outcomes in the Modern Era. JACC Hear Fail. 2020;8:131-40. https://doi.org/10.1016/j.jchf.2019.08.014. [ Links ]
15. Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state-of-the-art review from the Translational Research Committee of the Heart Failure Association-European Society of Cardiology. Eur Heart J. 2018;39:4243-54. https://doi.org/10.1093/eurheartj/ehy596. [ Links ]
16. Aimo A, Gaggin HK, Barison A, Emdin M, Januzzi JL Jr. Imaging, Biomarker, and Clinical Predictors of Cardiac Remodeling in Heart Failure With Reduced Ejection Fraction. JACC Heart Fail. 2019;7:782-94. https://doi.org/10.1016/j.jchf.2019.06.004. [ Links ]
17. Monmeneu JV, Bodí V, López Lereu MP, Sanchis J, Nuñez J, Chaustre F, et al. Análisis mediante resonancia magnética cardiaca del miocardio salvado tras infarto. Predictores e influencia en el remodelado adverso ventricular. Rev Esp Cardiol 2012;65:634-1. https://doi.org/10.1016/j.recesp.2012.01.024 [ Links ]
18. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, et al. Relationship between Infarct Size and Outcomes Following Primary PCI Patient-Level Analysis from 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674-83. https://doi.org/10.1016/j.jacc.2016.01.069. [ Links ]
19. San Miguel L, Goldschmidt E, Brisbin AK, Redruello M, Masoli OH. A new perspective on an old method: gated SPECT imaging for left ventricular contractile function assessment. J Nucl Cardiol. 2023;30:2658-65. https://doi.org/10.1007/s12350-023-03340-1. [ Links ]
20. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-6. https://doi.org/10.1161/01.CIR.0000072790.23090.41. [ Links ]
Received: October 20, 2023; Accepted: December 12, 2023











 texto em
texto em 



