Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cardiología
versão On-line ISSN 1850-3748
Rev. argent. cardiol. vol.92 no.1 Ciudad Autónoma de Buenos Aires mar. 2024 Epub 28-Fev-2024
http://dx.doi.org/10.7775/rac.es.v92.i1.20725
ORIGINAL ARTICLE
Impact of Extended Septal Miectomy on Echocardiographic Changes in Hypertophic Obstructivec Cardiomyopathy
1Familial Cardiomyopathy Clinic, Instituto Cardiovascular de Buenos Aires.
Background:
Extended septal myectomy (ESM) has proven to be a useful strategy to improve symptoms in patients with hypertrophic obstructive cardiomyopathy (HOCM).
Objectives:
The aim of this study was to analyze the impact of ESM on short and mid-term structural and functional echocardiographic parameters in patients with HCM and left ventricular (LV) outflow tract dynamic obstruction.
Methods:
Preoperative, immediate postoperative (1 month after surgery) and late postoperative (2 to 3 years) echocardiograms of patients undergoing ESM were analyzed.
Results:
A total of 94 patients with mean age of 57.6 ± 13.8 years underwent surgery. A significant reduction was observed in maximum septal thickness in the immediate postoperative period, which was sustained in the late postoperative period, and in atrial size in the immediate postoperative period, which deepened in the late postoperative period (p < 0.001). Intraventricular gradient at rest dropped from 49.2 to 6.4 mmHg (p < 0.001) and then to 4.6 mmHg (p=0.224) in the immediate and late periods and with Valsalva maneuver from 93.9 to 8.7 mmHg (p < 0.001), and then to 7.2 mmHg (p=0.226), respectively. Preoperative diastolic function was assessed as grade II in 58.5% of patients, decreasing to 51.7% in the immediate postoperative period and to 29% in the late postoperative period. In agreement with these results, a change was evidenced in the E/e´ ratio and pulmonary artery systolic pressure, degree of mitral regurgitation and left atrial dimensions.
Conclusion:
In this cohort of patients with HOCM, ESM was associated with a significant improvement in LV diastolic function, reduction in filling and pulmonary pressures and degree of mitral regurgitation, and left atrial reverse remodeling. It is possible that this combination of effects explains the clinical benefits of the intervention.
Key words: Hypertrophic cardiomyopathy; septal myectomy; Echocardiography
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a hereditary cardiac disease with complex genetic and phenotypic expression and an estimated prevalence of 1:500 in the general population. 1),(2
Approximately 70% of patients with HCM present some form of dynamic obstruction (HOCM) that contributes to the development of symptoms such as dyspnea and angina. In turn, sufficient evidence shows the impact of dynamic obstruction in the development of functional and structural changes at the cardiac level, as the increase in ventricular filling pressures, pulmonary pressure and left and right atrial dilation. 3),(4
Extended septal myectomy (ESM) has shown to be a useful strategy to improve symptoms and quality of life in patients with HOCM refractory to medical treatment. 3),(4),(5
The purpose of this study was to evaluate the impact of this surgery on the structural changes associated with dynamic obstruction.
The objective was to analyze the short and midterm impact of ESM in structural and functional echocardiographic parameters in patients with HCM and left ventricular (LV) outflow tract dynamic obstruction.
METHODS
Study design
An observational study was carried out on a prospective cohort of patients undergoing ESM from November 2011 to December 2022. It included patients with possibility of follow- up and a control echocardiogram in the immediate and late postoperative periods.
HCM diagnosis
The HCM diagnosis was performed according to international guideline recommendations, with the presence of increased LV wall thickness in one or more myocardial segments, determined by any imaging technique, in the absence of any other cardiac or systemic cardiac disease that justified it. In adults, a LV wall thickness ≥15 mm, or a septal/posterior wall thickness ratio >1.3 was considered to determine a diagnosis, and for first-degree relatives of a patient with HCM, a LV wall thickness ≥13 mm was contemplated for the diagnosis. 2),(3
The HOCM diagnosis was performed according to international guideline recommendations by Doppler echocardiography, when the peak gradient obtained spontaneously and/or using maneuvers (Valsalva, exercise) was ≥30 mmHg. 2),(3
Echocardiographic assessment
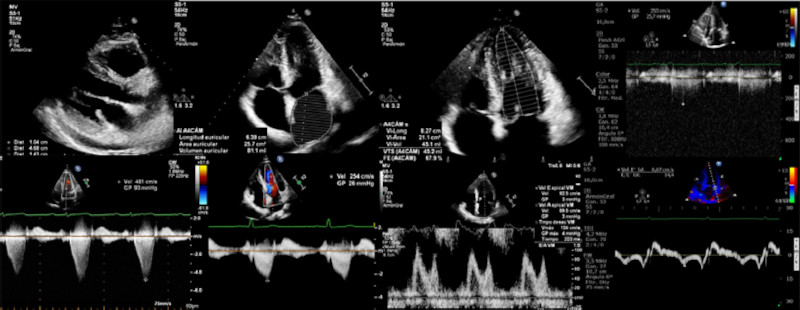
A two-dimensional ultrasound was used to measure myocardial maximum septal thickness, left atrial (LA) anteroposterior diameter in the long parasternal axis, and atrial area and volume in apical 4-chamber view. Left atrial volume was calculated using the area-length biplane method and the LA volume index using body surface area.
Left ventricular systolic function was quantified by Simpson’s rule, in apical 2-chamber and 4-chamber views. 6
The apical 4-chamber view with pulsed Doppler mitral flow at the mitral valve leaflet tips was used to determine peak early (E) and late (A) diastolic wave velocities, the E/A ratio and E wave deceleration time, and to classify diastolic dysfunction according to echocardiographic quantification guidelines into normal and grade I, II and III. Early lateral and medial mitral annulus peak diastolic velocities were analyzed with tissue Doppler to assess ventricular filling pressures, considering the ratio between mitral peak E wave velocity and average peak e’ wave velocity (E/e’). 7
According to international recommendations, diastolic function assessment in HCM patients was considered through an integral approach, taking into account all the echocardiographic parameters that include and consider as abnormal values an E/e’ ratio >14, indexed LA volume >34 ml/m2, difference in the pulmonary vein retrograde A wave duration - transmitral flow antegrade A wave duration >30 msec, and peak tricuspid regurgitant jet velocity >2.8 m/s. Normal LA pressure and grade I diastolic dysfunction was considered if < 50 % of these variables reached cut-off values, and elevated LA pressure and grade II diastolic dysfunction when > 50% of these variables did. In case of 50% discordance, the findings were not conclusive to estimate LA pressure. Grade III diastolic dysfunction was considered in the presence of a restrictive filling pattern and reduced Doppler tissue mitral annulus velocity (septal < 7 cm/s, and lateral < 10 cm/s). 7
Presence of mitral regurgitation was evaluated with color Doppler ultrasound, and qualitatively graded as mild, moderate and severe, and whether its mechanism was secondary to anterior systolic mitral valve motion, or as a consequence of a valve abnormality, such as prolapse, chordal rupture, mitral valve ring calcification or incorrect positioning of a papillary muscle, or mitral subvalvular apparatus abnormalities. 7
To assess pulmonary artery systolic pressure (PASP), peak tricuspid regurgitant jet velocity continuous Doppler was used, added to right atrial diastolic pressure, according to the inferior vena cava diameter and its percentage of inspiratory collapse. 7
The obstructive gradient was identified with color Doppler and quantified with continuous Doppler according to the Bernoulli formula, at rest and with Valsalva maneuver/ exercise. 7
The clinical preoperative, postoperative and follow-up evaluation of patients was performed by cardiologists of the Cardiomyopathy center, and functional class for dyspnea, according to the New York Heart Association (NYHA) classification, presence or absence of angina, syncope, family history of sudden death, cardiovascular risk factors and optimal medical treatment were established (Figure 1).
Follow-up
Transthoracic echocardiograms were performed in the following periods:
Preoperative period: up to 3 months before surgery
Immediate postoperative period: 1 month after surgery
Late postoperative period: between 2 and 3 years after surgery.
Statistical analysis
Quantitative variables are expressed as mean or median with their corresponding standard deviation (SD) or interquartile range (IQR) as appropriate. Categorical variables are presented as frequency and percentage. The Kolmogorov- Smirnov test (n ≥30) and the Shapiro-Wilk test (n < 30) were used to evaluate the normality of variables and the application assumptions of each of the tests used.
Chi-square, McNemar, sign, Wilcoxon or ANOVA tests were used as appropriate (2 samples or more, independent or paired)
A two-tailed p value < 0.05 was considered significant.
SPSS 19 (Statistical Package for the Social Sciences) was used to perform the analyses.
Ethical considerations
The study was carried out according to national ethical regulations (CABA law 3301), the national law of clinical research in human beings and the declaration of Helsinki, and was approved by the Institutional Teaching and Ethics Committee.
Patients signed an informed consent for the anonymous use of their personal data.
RESULTS
A total of 94 patients, with mean age 57.6±13.8 years, 53% female, underwent surgery during the study period, with a median follow-up of 3.3 years (IQR 1.1- 5.1) Table 1.
Table 1 Baseline population characteristics.
| Variables | n= 94 |
|---|---|
| Age (years), mean ±SD | 57.3 ± 13.2 |
| Female gender, n (%) | 50 (53.1) |
| HTN, n (%) | 59 (62.7) |
| Dyslipidemia, n (%) | 54 (57.4) |
| Diabetes, n (%) | 11 (11.7) |
| Smoking, n (%) | 21 (22.3) |
| Syncope, n (%) | 13 (13.8) |
| Angina, n (%) | 17 (18) |
| Coronary artery disase, n (%) | 19 (20.2) |
| Pharmacological treatment, n (%) | |
| Betablockers | 65 (69.1) |
| Verapamil | 2 (2.1) |
| Amiodarone | 23 (24.4) |
| Diuretics | 35 (36.1) |
| ARBs | 17 (18) |
| Family history of sudden death, n (%) | 11 (11.7) |
| Prior alcohol septal ablation, n (%) | 6 (6.3) |
| Prior ICD, n (%) | 11 (11.7) |
ARBs: Angiotensin receptor blockers; HTN: Hypertension; ICD: Implantable cardioverter defibrillator; SD: Standard deviation
All patients underwent ESM and 43 of them (45.7%) were also subjected to concomitant treatment of the mitral valve or the subvalvular mitral apparatus (9 underwent mitral valve repair, 26 mitral valve plication, 14 second order chord resection, 8 accessory muscle resection, and 3 mitral valve replacement). In addition, aortic valve replacement was performed in 3 patients (3.1%), coronary bypass surgery in 19 (20.2%) and Maze surgery in 17 (18%).
All patients presented complete left bundle branch block after the intervention, and 7.4% required definite pacemaker for complete atrioventricular block, in 5.3% of cases in patients with isolated septal myectomy. Two patients presented atrial fibrillation (AF) in the immediate preoperative and postoperative periods and 5 in the late postoperative period.
There were 3 (3.1%) interventricular septal defects related with the surgical procedure, and 30-day perioperative mortality was 1 patient (1.06%). Nine patients (9.5%) died during follow-up, 3 related to their underlying disease (3.2%)
Regarding functional class, 89% of patients were asymptomatic during the long-term follow-up.
Structural parameters showed a significant reduction in maximum septal thickness, from 21.3 mm in the preoperative period to 14 mm in the immediate postoperative period, (p < 0.001) which was maintained in the late postoperative period.
A significant reduction was observed in the left atrial size; its anteroposterior diameter decreased from 49.7 mm to 48.2 mm in the immediate postoperative period (p < 0.001) to 46.2 mm in the late postoperative period (p < 0.001). Left atrial volume indexed by body surface area decreased from 57.6 ml/m2 to 51.2 ml/m2 (p < 0.001) in the immediate postoperative period, and to 46.1 ml/m2 (p < 0.001) in the late postoperative period. Moreover, left atrial area decreased from 30.1 cm2 to 28.2 cm2 (p < 0.001) in the immediate postoperative period, and to 26.3 cm2 (p < 0.001) in the late postoperative period (Table 2 and Figure 2).
Table 2 Modification of echocardiographic parameters with the ESM
| Variable | Preoperative | Immediate POP | Late POP | p |
|---|---|---|---|---|
| Maximum thickness (mm), mean ±SD | 21.3 ± 3.9 | 14 ± 3 | 14 ± 3 | <0.001 |
| Gradient at rest (mmHg), mean ±SD | 49.2 ± 31.4 | 6.4 ± 9.3 | 4.6 ± 9.4 | <0.001 |
| Valsalva gradient (mmHg), mean ±SD | 93.9 ± 39.7 | 8.7 ± 13 | 7.2 ± 14 | <0.001 |
| LA volume (ml/m2), mean ±SD | 57.6 ± 18.4 | 51.2 ± 17.2 | 46.1 ± 16.5 | <0.001 |
| LA area (cm2), mean ±SD | 30.1 ± 5.9 | 28.2 ± 5.4 | 26.3 ± 5.6 | <0.001 |
| LA anteroposterior diameter (mm), (mean ±SD) | 49.7 ± 6 | 48.2 ± 5.8 | 46.2 ± 5.4 | <0.001 |
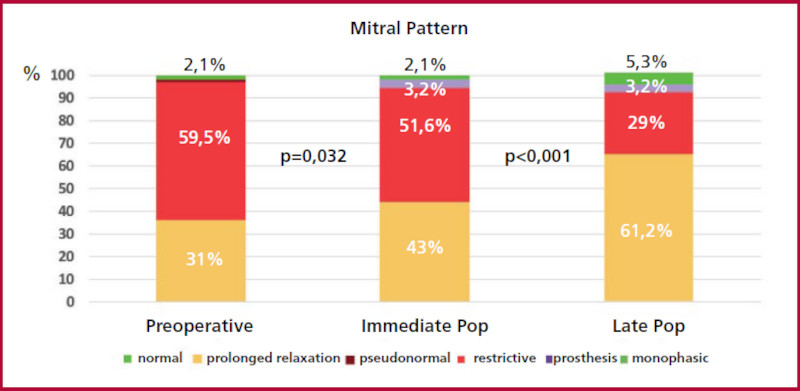
| Diastolic function, n (%) | ||||
| Normal | 0 | 0 | 1 (1.2) | |
| AF | 2 (2.1) | 2 (2.1) | 5 (5.3) | |
| Grade I | 36 (38.3) | 40 (43) | 57 (61.3) | <0.001 |
| Grade II | 55 (58.5) | 48 (51.7) | 27 (29) | |
| Grade III | 1 (1.1) | 0 | 0 | |
| Mitral valve prosthesis | 0 | 3 (3.2) | 3 (3.2) | |
| E/e´ ratio, mean ±SD | 15.7 ± 4.8 | 13 ± 3.3 | 11.1 ± 3.2 | <0.001 |
| LVEF (%), mean ±SD | 65 ± 6.2 | 59.9 ± 6.1 | 60 ± 6.8 | <0.001 |
| PASP (mmHg), mean ±SD | 32.9 ± 9.5 | 30.1 ± 7.9 | 27.5 ± 8.4 | <0.001 |
| Mitral regurgitation, n (%) | ||||
| None | 0 | 3 (3.2) | 3 (3.2) | |
| Mild | 44 (46.7) | 80 (87.9) | 80 (87.9) | <0.001 |
| Moderate | 38 (40.4) | 8 (8.7) | 8 (8.7) | |
| Severe | 12 (12.7) | 0 | 0 | |
AF: Atrial fibrillation; ESM: extended septal myectomy; LA: Left atrial; LVEF: Left ventricular ejection fraction; PASP: Pulmonary artery systolic pressure; POP: Postoperative; SD: Standard deviation
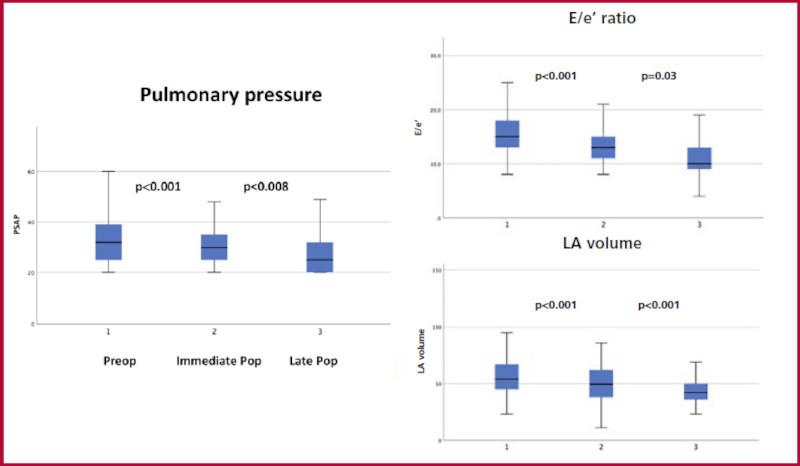
Fig. 2 Extended septal myectomy and changes in left atrial volume and E/e´ ratio. IQR: Interquartile range.LA: Left atrial.
Regarding functional parameters, the intraventricular gradient at rest decreased from 49.2 mmHg to 6.4 mmHg (p < 0.001), and then to 4.6 mmHg (p=0.224) in the immediate and late postoperative periods, and with the Valsalva maneuver from 93.9 mmHg to 8.7 mmHg (p < 0.001), and 7.2 mmHg (p=0.226), respectively. Diastolic function was assessed as grade II in 59.3% of patients in the preoperative period, in 51.6% in the immediate postoperative period, and in 29% in the late postoperative period (Figure 3). Similarly, a change in the E/e´ ratio and pulmonary artery systolic pressure was evidenced. Moderate to severe mitral regurgitation decreased from 53.1% in the preoperative period to 8.7% in the late postoperative period and pulmonary pressure from 32 mmHg to 27 mmHg, respectively. Left ventricular ejection fraction (LVEF) decreased from 65% to 60% (p < 0.001) in the immediate postoperative period and remained at 60% (p=0.953) in the late postoperative period.
DISCUSSION
The importance of the results obtained in this work is the significant impact of ESM on the different hemodynamic parameters studied by echocardiography and the clinical improvement of patients. We would like to highlight 4 findings.
First, from a structural point of view, a reduction was evidenced in the LA size measured in all its forms, which appears to be intensified with the passage of time. The probable mechanisms that we understand are involved in this phenomenon are the reduction of mitral regurgitation and of LV filling pressure. In relation to the first, patients undergoing surgery for isolated mitral regurgitation usually show this phenomenon, correlated in the case of mitral valve repair surgery with the degree of residual mitral regurgitation. Regarding filling pressures, the reduction of the dynamic gradient has a verified impact at the level of end-diastole pressure and contributes to this phenomenon. From a prognostic viewpoint, LA size is an independent risk factor for adverse outcomes in patients with HCM, and increased LA volume is associated with an increased risk of atrial fibrillation, thromboembolic stroke, heart failure and cardiovascular mortality, which appears to represent much more than a simple structural change. 8),(9),(10),(11),(12),(13),(14),(15),(16),(17),(18 Our findings have been previously reported by Tower-Rader et al., who in a review of 25 patients observed a significant decrease in atrial volume 2 years after myectomy. 19 Similarly, Finocchiaro et al. demonstrated a marked reduction of LA volume in a cohort of 40 patients undergoing septal reduction therapy (myectomy or alcohol septal ablation). 20 In a cohort of 44 operated-on patients, Kyung Eun Ha et al. observed a reduction in atrial volume and increased strain of this chamber, consistent not only with the reduction in size but with an improvement in fiber function. 21
Second, echocardiographic “hemodynamic” assessment showed a reduction in the E/e´ ratio and changes in the transmitral filling pattern. These modifications seem to be related with the reduction in LV end diastolic pressure, secondary to the significant reduction that the procedure achieved in the dynamic left ventricular outflow tract gradients both at rest and with Valsalva maneuver. Evidence of this is that the changes are noticeable in the immediate postoperative period. Moreover, both variables tend to improve even more in the late postoperative period, a fact that could be explained by the gradual recovery in diastolic function produced by the relief of the obstruction. This has been also reported by others, such as Nguyen et al, who found in a cohort of 656 patients an improvement in the E/e' ratio, which occurred in the early postoperative phase and was maintained in the long-term follow-up. 22 There is evidence that correlates dynamic obstruction with the development of myocardial ischemia and interstitial fibrosis, so we hypothesize that this relief could be generating a retrograde phenomenon. 23),(24
Third, the LVEF drop in the immediate postoperative period could be associated with the impact of the pump used for cardiopulmonary bypass during surgery, but above all to the development of left bundle branch block due to the myectomy. This last phenomenon causes mechanical dyssynchrony and a drop in ejective volume, without ruling out that it has an impact on the reduction of the dynamic gradient. The analysis of the late postoperative period shows a numerical though not significant recovery, requiring greater follow-up for analysis. 23),(24),(25),(26 Along the same line, Ha et al. observed a relationship between postmyectomy QRS duration and the drop in LVEF, accompanied by a reduction in non-septal LV longitudinal strain. 21
Fourth, patients showed a reduction in echocardiographic assessed pulmonary artery systolic pressure that deepened over time. This fact shows that changes in left filling pressures and improvement in diastolic function have an impact on the pulmonary circuit and right chambers, which probably contributes to explaining the clinical improvement of patients. 25),(26
Limitations
The number of patients included may decrease the reliability of several of the data obtained. However, the consistency of the findings suggests a direct relationship between the intervention and the observed changes. We also understand the limitations of the methods to accurately estimate ventricular filling pressures in this scenario, though there is sufficient information to consider that changes in these variables are associated with improvement in diastolic function.
CONCLUSIONS
In this cohort of patients with HOCM, ESM was associated with a significant improvement in LV diastolic function, reduction in filling and pulmonary pressures and degrees of mitral regurgitation, and reverse remodeling of the left atrium.
It is possible that this combination of effects explains the clinical benefits of the intervention.
BIBLIOGRAFÍA
1. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrohic cardiomyopathy in general population of young adults. Circulation 1995;92:785-9. https://doi.org/10.1161/01.CIR.92.4.785 [ Links ]
2. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. Guía de práctica clínica de la ESC 2014 sobre el diagnóstico y manejo de la miocardiopatía hipertrófica. Rev Esp Cardiol 2015;68:63-52. https://doi.org/10.1016/j.recesp.2014.12.001 [ Links ]
3. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:783-831. https://doi.org/10.1161/CIR.0b013e318223e2bd [ Links ]
4. Maron MS, Olivotto I, Betochi S, Casey SA, Lesser JR, Losi MA et al. Effect of left ventricular outflow tract obstruction on clínical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295- 303. https://doi.org/10.1056/NEJMoa021332 [ Links ]
5. Maron BJ. Controversies in cardiovascular medicine. Surgical myectomy remains the primary treatment option for severely symptomatic patients with obstructive hypertrophic cardiomyopathy. Circulation 2007;116:196-206. https://doi.org/10.1161/CIRCULATIONAHA.107.691378 [ Links ]
6. Mitchel C, Rahko PS, Blauwet L, Canaday B, Finstuen J, Foster C, et al. Guidelines for performing a Comprenhensive Transthoracic Echocardiographic Examination in Adults. JASE 2019;32:1-62. https://doi.org/10.1016/j.echo.2018.06.004 [ Links ]
7. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. https://doi.org/10.1016/j.echo.2016.01.011 [ Links ]
8. Morrow AG, Fogarty TJ, Hannah H III, Braunwald E. Operative treatment in idiopathic hypertrophic subaortic stenosis. Techniques and results of postoperative clinical and hemodynamic assessments. Circulation 1968;37:589-96. https://doi.org/10.1161/01.CIR.37.4.589 [ Links ]
9. Sherrid MV, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy: echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. Ann Thorac Surg 2003;75:620-32. https://doi.org/10.1016/S0003-4975(02)04546-0 [ Links ]
10. Messmer BJ. Extended myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg 1994;58:575-7. https://doi.org/10.1016/0003-4975(94)92268-3 [ Links ]
11. Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: Septal myectomy for obstructive hypertrophic cardiomyopathy- the Mayo Clinic experience. Nat Clin Pract Cardiovasc Med 2007;4:503-12. https://doi.org/10.1038/ncpcardio0965 [ Links ]
12. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2005;46:470-6. https://doi.org/10.1016/j.jacc.2005.02.090 [ Links ]
13. Fifer MA, Vlahakes GJ. Management of symptoms in hypertrophic cardiomyopathy. Circulation 2008;117:429-39. https://doi.org/10.1161/CIRCULATIONAHA.107.694158 [ Links ]
14. Mogelvang R, Sogaard P, Pedersen SA, Olsen NT, Marott JL, Schnohr P, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679-85. https://doi.org/10.1161/CIRCULATIONAHA.108.793471 [ Links ]
15. Casabé JH, Acunzo R, Fernández A, Gabay J, Galizio N, Hita A, y cols. Consenso Argentino de diagnóstico y tratamiento de la Miocardiopatía Hipertrófica. Rev Argent Cardiol 2017;85(Suplemento 2) [ Links ]
16. Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol 2006;47:1018-23. https://doi.org/10.1016/j.jacc.2005.08.077 [ Links ]
17. Nistri S, Olivotto I, Betocchi S, Losi MA, Valsecchi G, Pinamonti B, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian Registry for Hypertrophic Cardiomyopathy). Am J Cardiol. 2006;98:960-5. https://doi.org/10.1016/j.amjcard.2006.05.013 [ Links ]
18. Losi MA, Betocchi S, Barbati G, Parisi V, Tocchetti CG, Pastore F, et al. Prognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22:76-81. https://doi.org/10.1016/j.echo.2008.11.001 [ Links ]
19. Tower-Rader A, Furiasse N, Puthumana JJ, Kruse J, Li Z, Andrei AC et al. Effects of septal myectomy on left ventricular diastolic function and left atrial volume in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2014;1141568-72. https://doi.org/10.1016/j.amjcard.2014.08.029 [ Links ]
20. Finocchiaro G, Haddad F, Kobayashi Y, Lee D, Pavlovic A, Schnittger I et al. Impact of Septal Reduction on Left Atrial Size and Diastole in Hypertrophic Cardiomyopathy. Echocardiography. 2016;33:686-94. https://doi.org/10.1111/echo.13158 [ Links ]
21. Ha KE, Choi KU, Lee HJ, et al. Effects of septal myectomy on left atrial and left ventricular function in obstructive hypertrophic cardiomyopathy. ESC Heart Fail. 2023;10:2939-47. https://doi.org/10.1002/ehf2.14481 [ Links ]
22. Nguyen A, Schaff HV, Nishimura RA, Dearani JA, Geske JB, Lahr BD. Determinants of Reverse Remodeling of the Left Atrium After Transaortic Myectomy. Ann Thorac Surg. 2018;106:447-453. https://doi.org/10.1016/j.athoracsur.2018.03.039 [ Links ]
23. Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;116:2702-8. https://doi.org/10.1161/CIRCULATIONAHA.107.698985 [ Links ]
24. Maciver DH. A new method for quantification of left ventricular systolic function using a corrected ejection fraction. Eur J Echocardiogr. 2011;12:228-34. https://doi.org/10.1093/ejechocard/jeq185 [ Links ]
25. Desai MY, Bhonsale A, Smedira NG, Naji P, Thamilarasan M, Lytle BW, et al. Predictors of long-term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation 2013;128:209-16. https://doi.org/10.1161/CIRCULATIONAHA.112.000849 [ Links ]
26. Schaff HV, Said SM. Transaortic Extended Septal Myectomy for Hypertrophic Cardiomyopathy. Operative Techn Thorac Cardiovascr Surg 2012;17:238-50. https://doi.org/10.1053/j.optechstcvs.2012.04.002 [ Links ]
Received: November 11, 2023; Accepted: January 09, 2024











 texto em
texto em