Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cardiología
versão On-line ISSN 1850-3748
Rev. argent. cardiol. vol.92 no.1 Ciudad Autónoma de Buenos Aires mar. 2024 Epub 28-Fev-2024
http://dx.doi.org/10.7775/rac.es.v92.i1.20728
Articles
Implications of Artificial Intelligence in Intravascular Imaging Methods
1Department of Interventional Cardiology , Hospital Italiano de Buenos Aires Argentina
2Department of Interventional Cardiology , Hospital Clínico San Carlos, Madrid, España
3National Laboratory for Scientific Computing, LNCC/MCTI, Petrópolis, RJ, Brazil
4National Institute of Science and Technology in Medicine Assisted by Scientific Computing, INCT-MACC, Petrópolis, RJ, Brazil.
5 5Interventional Cardiology , MedStar Washington Hospital Center, Washington, Estados Unidos
Percutaneous coronary intervention (PCI) is one of the primary revascularization strategies in patients with coronary artery disease (CAD). Several studies support the use of intravascular imaging methods to optimize PCI. However, these methods are underutilized in contemporary clinical practice and face challenges in data interpretation. Therefore, the incorporation of artificial intelligence (AI) is seen as an attractive solution to promote and simplify their use.
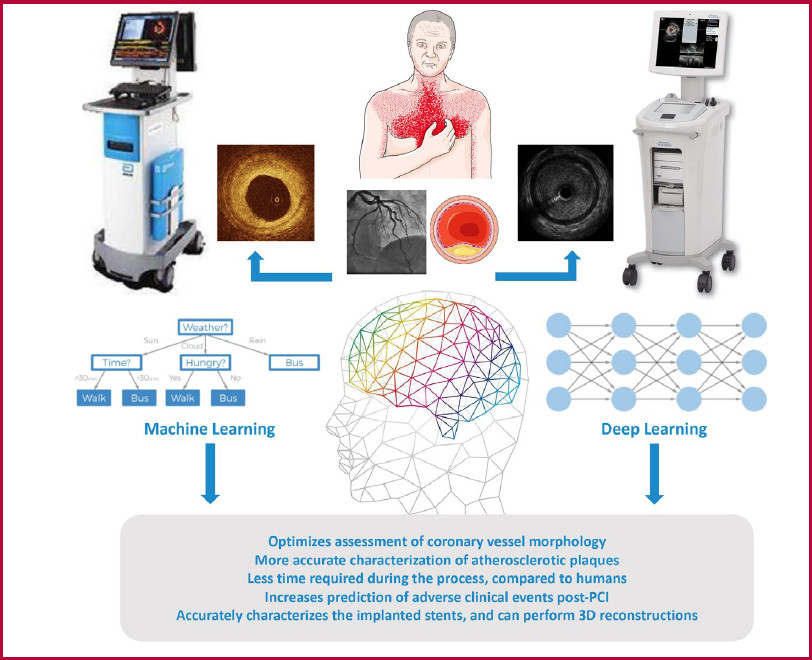
AI can be defined as a computer program that mimics the human brain in its ability to collect and process data. Machine learning is a sub-discipline of AI that involves the creation of algorithms capable of analyzing large datasets without making prior assumptions, while deep learning focuses on the construction and training of deep and complex artificial neural networks. The incorporation of AI systems to intravascular imaging methods improves the accuracy of PCI, reduces procedure duration, and minimizes interobserver variability in data interpretation. This promotes their wider adoption and facilitates their use. The aim of this review is to highlight how current AI-based systems can play a key role in the interpretation of data generated by intravascular imaging methods and optimize PCI in patients with CAD.
Key words: Artificial Intelligence; Percutaneous Coronary Intervention; Intravascular Imaging
INTRODUCCIÓN
Despite the remarkable advances achieved in preventive strategies and therapeutic approaches in recent decades, atherosclerotic coronary artery disease (CAD) remains the leading cause of morbidity and mortality worldwide. 1 Although percutaneous coronary intervention (PCI) is recognized as a fundamental therapeutic strategy for endovascular revascularization in patients with CAD, its benefit is limited by the need for prior and accurate interpretation of data obtained by diagnostic coronary angiography, which has significant limitations in the assessment of vascular involvement due to its characteristics. In this context, and in line with advances in pharmacological treatment strategies, technologies based on imaging and intravascular physiology have been developed and refined to assess the risk of patients with CAD and to improve their treatment.
So far, several studies have supported the optimization of PCI with stent implantation guided by intravascular imaging methods, such as intravascular ultrasound (IVUS) or optical coherence tomography 10. These methods offer considerable benefits by significantly improving stent implantation and reducing the incidence of adverse clinical events during follow-up. 2,3,4,5 However, the use of these optimization techniques is currently limited, possibly due to their cost to healthcare systems, the need for operator experience to accurately interpret the data obtained, and the fact that they can significantly increase the overall procedure time. In this context, the incorporation of artificial intelligence (AI) as a tool to facilitate and simplify the use of intravascular imaging and the interpretation of the information obtained is emerging as an attractive strategy. The aim of this review is to highlight how current AI-based systems can play a key role in the interpretation of data generated by intravascular imaging techniques, and thus improve optimization of PCI in patients with CAD.
RATIONALE FOR ARTIFICIAL INTELLIGENCE
AI can be defined as a computer program that resembles the human brain in its ability to collect and process data. 6 This concept was first coined in 1956 during the Dartmouth Summer Research Project 7 and generated explosive interest in the 1970s to be implemented in biomedical sciences. 8
Machine learning (ML) is a sub-discipline of AI that involves the creation of algorithms capable of analyzing large datasets without making prior assumptions and learning to identify rules and patterns among variables for prediction and classification. 9 The versatility and great potential of these algorithms are due to their ability to incorporate a wide set of variables from various medical modalities, ranging from clinical parameters to two- and threedimensional imaging data, taking into account the multidimensional nonlinear interactions between them. ML approaches can be broadly classified into three categories: supervised learning, unsupervised learning, and semi-supervised learning. 10 Supervised learning is the most common approach in ML, where the system establishes associations from training data obtained from examples that already have an outcome identified by a specialist. The algorithm embeds the specialist's knowledge within the model. In this method, from the given input features, the system outputs a suitable regression or classification analysis of clinical and imaging data. On the other hand, in situations where training data is unavailable or insufficient, unsupervised learning is used to discover hidden structure of data. 10 Finally, semisupervised learning begins with a small set of labeled data and augments the training data size by gradually labeling unlabeled data. 10 (Table 1).
Table 1 Machine Learning categories.
| Category | Description | Subtype | Examples |
|---|---|---|---|
| Supervised learning | Labelled data and results | Classification: Uses an algorithm to assign data into specific categories, reaching conclusions on how to appropriately label those categories within the dataset | Logistic regression, Bayesian networks, Random Forest, Ridge regression, Elastic Net regression, LASSO (Least Absolute Shrinkage and Selection Operator) regression and artificial neural networks. |
| Regression: Analyzes the relationship between dependent and independent variables. It is commonly used to make projections | |||
| Unsupervised learning | Detects crucial realtionships and similarites in unlabelled datasets | Clustering: Groups unlabeled data based on their similarities or differences | Hierarchical clustering, K-means clustering and Principal component analysis. |
| Dimensionality reduction: Reduces the number of data inputs to a manageable size while also preserving the integrity of the dataset; used when the number of features, or dimensions, in a given dataset is too high | |||
| Semi- supervised learning | A combination of supervised learning and unsupervised learning | It includes both labeled and unlabeled results and classes, and is used in image and speech recognition systems. | |
| Reinforcement learning | Based on behavioral psychology, uses a reward function | Utilizes specific reward criteria and is used in medical imaging, analysis and disease detection. |
Deep learning (DL) is a sub-discipline of ML that focuses on the construction and training of deep and complex artificial neural networks. These networks are designed to mimic the learning process of the human brain. Unlike conventional neural networks, which may have only a few hidden layers, deep neural networks have multiple hidden layers, which allow the network to learn hierarchical and abstract features of input data, which make them especially effective for image processing tasks. Convolutional neural networks (CNN) represent a specialized type of artificial neural network specially designed for processing and analyzing the spatial structure of data that has a grid-like topology, such as an image. Several studies have used CNN to automatically characterize atherosclerotic plaques present in the coronary arteries, employing a variety of approaches. 11,12,13
Thus, a suitably trained AI system can comprehensively analyze diverse data and provide a diagnosis by interpreting information in a novel way. This makes it an appealing tool for its incorporation into image-based biomedical techniques to improve risk prediction and personalize clinical decisions.
IMPLEMENTATION OF AI IN INTRAVASCULAR IMAGING METHODS
Visualization of the cross-sectional anatomy of the coronary arteries using intravascular imaging methods has high sensitivity in identifying and characterizing atherosclerotic plaques by extracting tissue-specific parameters from the backscattered ultrasound signal. 14,15 In this context, when considering IVUS as a strategy to optimize revascularization by PCI, multiple clinical studies have shown that it reduces adverse clinical events at follow-up compared to standard angiographic guidance. 2,3,4
Limitations of IVUS include its limited axial resolution, which makes it difficult to accurately identify thin-cap fibroatheroma (TCFA), and its limited lateral resolution which may hinder the proper identification of coronary vascular dissections and atheroma plaques, as well as the correct positioning of stent struts. Considering these limitations, ML-based algorithms offer opportunities to optimize IVUS performance.
OCT has an inherent advantage in the morphological analysis of atherosclerotic plaques due to its high spatial resolution. 16 Compared to IVUS, OCT offers superior resolution, enabling clear visualization of thin fiber layers. However, its ability to penetrate tissues is limited, which lowers its capacity in detecting plaque size, large lipid cores, and external elastic layers. 17 It is important to note that the human eye has limitations, which can lead to the omission of a significant amount of data in the images. Therefore, there is an urgent need to introduce new optimization technologies in intravascular imaging methods to improve diagnostic efficiency and accuracy. AI constitutes a promising option within this framework.
In this scenario, there are DL methods based on adversarial attacks on CNN learning that could be used to transfer knowledge between IVUS and OCT. For example, images acquired with OCT and IVUS could be used to train AI models to increase the resolution of IVUS images. Similarly, IVUS images could be used to train AI models to complement the information of deep atherosclerotic plaque in OCT images. (Figure 1)
INCORPORATION OF AI IN PRE-PROCEDURAL ASSESSMENT
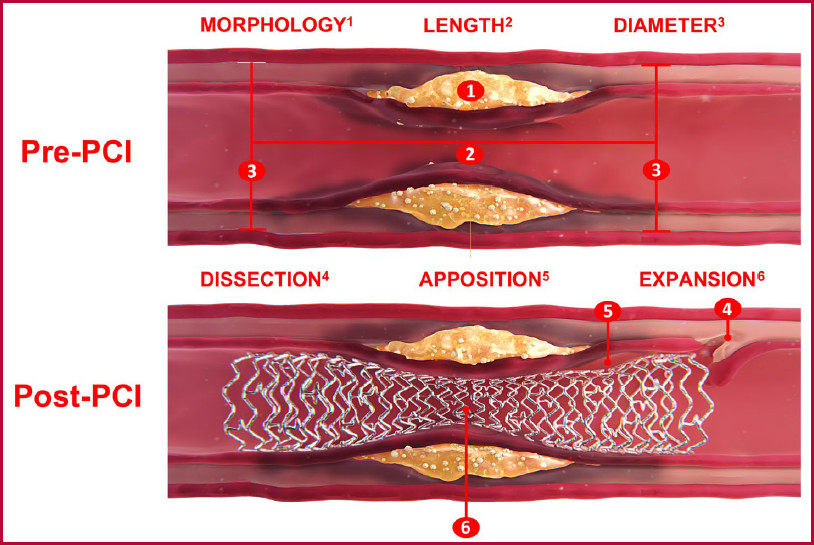
In the assessment before PCI, it is crucial to perform a thorough characterization of the vascular anatomy and atherosclerotic plaque using intravascular imaging methods to adequately select the endovascular technique to be used and the dimensions of the stent to be implanted. In this context, it is essential to identify the following characteristics before PCI, including the morphology and length of the atherosclerotic plaque, the diameter of the coronary vessels, and the predicted outcome of the intervention (Figure 2).
Several studies have explored the feasibility of implementing AI systems to improve accuracy, reproducibility, and speed in the analysis of intravascular imaging methods. In this setting, a multicenter observational study evaluated the accuracy and consistency of an AI system-based approach for automatic plaque characterization using OCT. 18 A post hoc analysis of OCT pullbacks from cohorts of patients with CAD participating in five different clinical trials was performed, dividing these data into two groups: training data set and testing data set. Thus, quantification of plaque burden using a CNN-based DL system for OCT analysis correlated very well with conventional manual pullback measurements (coefficient of determination R2 = 0.98, p < 0.001). 18 Furthermore, through an external validation process that included OCT pullbacks different from those previously analyzed, the CNN-based DL system demonstrated an overall diagnostic accuracy of 86.6% (95% CI: 83.7-89.1). The software performed the best in fibrous plaques (97.6%; 95% CI: 93.4-99.3%), followed by lipidic plaques (90.5%; 95% CI: 85.2-94.1%) and calcifications (88.5%; 95% CI: 82.4-92.7%). 18 Of note, one of the challenges associated with this type of study is the lack of histological validation, since the initial classification is often based on expert observations.
The incorporation of AI models in the characterization of the coronary arteries by means of OCT has improved the interpretation of atherosclerotic plaques and increased the predictive ability of adverse clinical events during follow-up. In this context, a retrospective observational study analyzed OCT pullbacks in the nonculprit vessel of patients with acute coronary syndrome. 19 In this analysis, AI was used to determine the optical flow ratio (OFR) as a surrogate parameter of coronary physiology derived from OCT, as well as to identify the lipid-to-cap ratio (LCR), a novel morphologic OCT index that integrates the lipid burden of the atherosclerotic plaque and the cap thickness over the diseased segment. 19 In this regard, determination of OFR and LCR using an AI system demonstrated superior discriminatory ability to predict nonculprit vessel-related major adverse cardiovascular events (NCV-MACE) compared with predictions based on minimum luminal area (MLA) determination and identification of TCFA. The combination of LCR > 0.33 and OFR ≤ 0.84 resulted independent risk factors for NCV-MACE during follow-up. 19 Table 2 presents studies in which AI was used as a strategy to optimize OCT.
Table 2 Studies using artificial intelligence as a strategy to optimize optical coherence tomography.
| Author | Year | Objetive | Algorithm used | Resultado |
|---|---|---|---|---|
| Athanasiou LS, et al. 20 | 2011 | To extract a set of features from grayscale OCT images and employ them to classify the atherosclerotic plaque using an ML algorithm. | Random Forest | The ML algorithm associated with OCT accurately characterized the atherosclerotic plaques |
| Wang Z, et al. 21 | 2012 | To propose and validate an OCT-based ML method to analyze fibrous cap volume to identify vulnerable atherosclerotic plaques. | Dynamic Programming | The proposed ML method is fast, accurate and robust, and can be used in future studies to further characterize atherosclerotic plaques. |
| Ughi GJ, et al. 22 | 2013 | To develop and validate an ML algorithm to optimize the characterization of atherosclerotic plaques by OCT. | Random Forest | The ML algorithm provided accurate characterization of atherosclerotic plaques. |
| Xu M, et al. 23 | 2014 | To propose a ML algorithm for automatic detection of atherosclerotic plaques using OCT | Support Vector Machine | The proposed ML system is accurate and stable for the detection of atherosclerosis by OCT. |
| Wang Z, et al. 24 | 2014 | To examine the quantitative tissue properties that can differentiate plaque erosion from intact fibrous plaques and to develop a computeraided classification model for in vivo diagnosis of plaque erosion | Regresión logística | The quantitative logistic regression model can be used to improve the diagnostic accuracy for plaque erosion in vivo using OCT. |
| Shalev R, et al. 25 | 2016 | To develop an ML method automatic classification of calcium in OCT images. | Filtering (filtrado) de regiones para la extracción de sub-imágenes (SIs) | The ML algorithm presents high accuracy for characterization of coronary artery calcium in OCT, images which could be used in 'real time'. |
| Rico-Jime nez JJ, et al. 26 | 2016 | To present a novel computational method for automated IV-OCT plaque characterization | A-line Modeling | The ML algorithm presents high accuracy for characterization of atherosclerotic plaques. |
| Xu M, et al. 27 | 2017 | To propose a DL model for the identification and characterization of fibroatheromas in OCT images. | AlexNet, GoogLeNet, VGG-16, VGG-19 | The DL system provides a highly accurate characterization of fibroatheromas |
| Shi P, et al. 28 | 2018 | To develop a DL-based model to identify vulnerable atherosclerotic plaques in OCT images. | Fully CNN, Deep CNN | The DL method presented high accuracy for characterizing vulnerable atherosclerotic plaques. |
| Guo X, et al. 29 | 2018 | To create a DL algorithm to characterize atherosclerotic plaque components and quantify fibrous cap thickness. | Least Squares Support Vector Machine (LS-SVM) | The segmentation method base on LS-SVM provided accurate characterization of fibrous cap thickness. |
| Kolluru C, et al. 30 | 2018 | To develop neural network-based methods for classifying plaque types in OCT images. | CNN, ANN | The DL model presented high accuracy for classifying atherosclerotic plaque subtypes. |
| He S, et al. 31 | 2018 | To analyze the performance of a DL model for characterization of atherosclerotic plaques in OCT images. | CNN | The DL algorithm demonstrated high accuracy to characterize atherosclerotic plaques. |
| Lee J, et al.32 | 2019 | To develop a DL system for automatic segmentation of atherosclerotic plaques based on OCT images. | SegNet | The DL algorithm showed high accuracy for segmentation of atherosclerotic plaques. |
| Prabhu D, et al. 33 | 2019 | To develop ML methods to identify fibrolipidic and fibrocalcific A-lines in OCT images. | Support Vector Machine | The proposed classification algorithm is suitable for automated OCT plaque classification and segmentation |
| Liu R, et al.34 | 2019 | To improve the detection quality of vulnerable plaque using a DL algorithm associated with OCT. | Deep CNN | The proposed DL algorithm has high accuracy to detect vulnerable plaques in OCT images. |
| Johnson KW, et al.35 | 2019 | To use a ML algorithm and transcriptomic data to estimate the predict if fibrous cap thickness will increase in response to statin therapy | Elastic net K top scoring pair | A ML algorithm using transcriptomic models could predict increase in fibrous cap thickness as a result of statin treatment. |
| Baruah V, et al.36 | 2020 | To determine the feasibility of a histologyvalidated virtual OCT (VH-OCT) algorithm using AI. | CNN | "This note is the first report to classify automatically tissue components with AI based on histological validation and extension into in vivo patient images." |
| Lee J, et al.37 | 2020 | To develop an ML algorithm for characterization of atherosclerotic plaques using a hybrid learning approach. | Deep CNN | The proposed algorithm could accurately characterize the atherosclerotic plaques. |
| Lee J, et al.38 | 2020 | To develop an automated, two-step DL approach for characterizing coronary calcified plaque | CNN, SegNet | Compared to the standard approach, the two-step DL system is highly accurate for characterization of atherosclerotic plaques in OCT images. |
| Cha JJ, et al.39 | 2020 | To evaluate the usefulness of ML FFR based on OCT and compare it with wire-based FFR. | Random Forest | OCT-based ML-FFR has good correlation with wireguided FFR. |
| Chu M, et al.18 | 2021 | To develop and validate a DL algorithm for characterization of atherosclerotic plaques in OCT images. | CNN | The DL algorithm for automatic characterization of atherosclerotic plaques in OCT images offers provides excellent diagnostic accuracy in both internal and external validation. |
| Balaji A, et al.40 | 2021 | To develop a DL algorithm for automatic coronary artery segmentation in OCT images. | DeepCap | The AI system linked to OCT demonstrated high accuracy for automatic segmentation of coronary Arteries. |
| Yin Y, et al.41 | 2021 | To develop DL algorithm for characterization of atherosclerotic plaques in OCT images. | TwopathCNN | A proposed DL algorithm presented higher accuracy for the characterization of atherosclerotic plaques, compared with conventional DL algorithms. |
| Li C, et al.42 | 2021 | To develop a DL algorithm for automatic quantification of coronary artery calcium in OCT images. | DenseNet, CNN | The proposed DL model presents high accuracy for discriminating coronary artery calcium in OCT Images. |
| Yang G, et al.43 | 2021 | To develop a DL model to automatically analyze stents with both thin (≤ 0.3 mm) and very thick (> 0.3 mm) tissue coverage, and an algorithm to accurately analyze stent area for vessels with multiple stents. | CNN | The proposed model can accurately detect stent struts with very thick tissue coverage and analyze stent areas in vessels implanted with multiple stents |
| Hong H, et al.19 | 2022 | To analyze the role of AI for predicting major adverse cardiovascular events by determining the plaque morphology and coronary artery physiology in OCT images. | Deep CNN | The study identified a novel DL algorithm to characterize the morphology and physiology of atherosclerotic plaques, which can predict the risk of adverse cardiovascular events. |
| Sun H, et al.44 | 2022 | To develop a DL-based AI method for fully automated detection of atherosclerotic plaque erosion. | Mask RCNN with convexity and curvature, Support Vector Machine | The developed DL algorithm presented good ability to discriminate atherosclerotic plaque erosion in OCT Images. |
| Wu P, et al.45 | 2023 | To analyze whether OCT and IVUS images can assist each other in stent 3D reconstruction | F1-score | Using AI systems, intravascular images of OCT and IVUS can provide reciprocal assistance to each other in stent 3D reconstruction. |
AI: artificial intelligence; ANN: artificial neural network; CNN: convolutional neural network; DL: deep learning; FFR: fractional flow reserve; IVUS: intravascular ultrasound; ML: machine learning; OCT: optical coherence tomography
IVUS is one of the main intravascular imaging methods for assessing coronary artery calcium, a predictor of adverse cardiovascular events and long-term mortality. 46 One of its limitations is the lack of an automated quantification system for coronary artery calcium. However, this limitation could be overcome by implementing AI systems that provide objective and reproducible automated quantification that correlates with validated coronary artery calcium scores. 47 In this context, a single-center retrospective cohort study evaluated the ability of an AI algorithm trained with IVUS imaging data to quantify coronary artery calcium and its association with adverse clinical events. 48 An IVUS calcium score (ICS) based on an AI model was shown to have a high predictive value for the development of adverse clinical events during follow-up. There was a 51% increased risk of adverse events in patients with an ICS ≥ 85. This ICS represents the first validated coronary calcium score associated with adverse clinical events since the introduction of computed tomography-derived calcium scoring. 48,49 AI systems have the remarkable ability to distinguish between various coronary artery calcium patterns, such as calcium in the artery wall and calcified nodule. (Table 3)
Table 3 Studies using artificial intelligence as a strategy to optimize intravascular ultrasound.
| Author | Year | Objetive | Results | Algoritmo |
|---|---|---|---|---|
| Sheet D, et al. 50 | 2014 | To develop an innovative ML-based technique (SDH) to automatically characterize morphology in IVUS images | Random Forest | SDH is highly consistent with traditional histology in characterizing calcification, fibrotic tissues and lipids. |
| Kim GY, et al. 51 | 2018 | To develop a classification model based on virtual histology to characterize atherosclerotic plaque components in fibrous tissue, fibro-fatty tissue, necrotic core, and dense calcium. | Multi-level classification model consisting of three different nets | The proposed method showed high accuracy for classifying all types of plaques |
| Bae Y, et al. 52 | 2019 | To develop ML models to predict optical coherence tomography (OCT) thin-cap fibroatheroma (TCFA). | ANN, Support Vector Machine , Naïve Bayes | The presence of OCT-TCFA was predicted with high accuracy by ML algorithms. |
| Jun TJ, et al. 53 | 2019 | To identify the most accurate method to classify TCFA using various ML classifiers. | Feed-forward neural network(FNN), K-nearest neighbor (KNN), Random Forest, CNN | The CNN classifier performed best, while FNN, KNN and Random Forest classifiers were found to be similar to the physician's TCFA diagnostic criteria. |
| Wang L, et al. 54 | 2020 | To identify the most accurate ML classifier to predict atherosclerotic plaque vulnerability change as determined by IVMP. | Generalized linear mixed regression model (GLMM), Support vector machine , Random Forest | MPVI was the best single risk factor using both GLMM and Random Forest while plaque area was the best using SVM. |
| Ziemer PG, et al.55 | 2020 | To evaluate the utility of a novel, automated ML system to segment the lumen boundary in IVUS datasets. | Multi-frame convolutional neural network | The proposed ML algorithm is suitable to effectively segment the lumen boundary in IVUS scans, reducing time required and need for manual delineation. |
| Lee JG, et al. 56 | 2020 | To determine the usefulness of AI algorithms for identifying functionally significant coronary stenoses (FFR ≤0.80 | L2 penalized logistic regres sion, ANN, Random Forest, AdaBoost, CatBoost, Support Vector Machine | IVUS-based ML algorithms showed good diagnostic performance for identifying ischemiaproducing lesions. |
| Cho H, et al. 57 | 2021 | To develop IVUS-based ML algorithms for classifying attenuation and calcified plaques. | EfficientNet | The ML algorithm for plaque characterization accurately identifies high-risk coronary artery lesions. |
| Neleman T, et al.48 | 2021 | To develop and validate an ML algorithm to automatically quantify coronary calcifications in IVUS images | Support Vector Machine | The IVUS calcium score calculated by an ML algorithm was strongly associated with the longterm risk of major adverse cardiac events. |
| Min HS, et al. 58 | 2021 | To develop pre-procedural IVUS-based DL models for predicting the occurrence of stent underexpansion. | CNN, eXtreme Gradient Boosting (XGBoost) | Los algoritmos de DL predijeron con gran precisión (94%) la infraexpansión del stent. |
| Bass RD, et al. 59 | 2022 | To compare the performance of human readers to the ML algorithm and against the readings from a Core Laboratory for coronary vessel segmentation. | Multi-frame convolutional neural network | Similar assessment of segmentation performed by humans, ML algorithm and core lab, with machines being more time efficient. |
| Bajaj R, et al. 60 | 2022 | To train and assess the efficacy of a ML classifier for plaque component classification that relies on IVUS echogenicity and NIRS-signal. | Algoritmo J48 | The combination of echogenicity with NIRS-signal appears capable of overcoming limitations of echogenicity. |
| Wissel T, et al. 61 | 2022 | To propose a fully data-driven strategy to longitudinally detect and subsequently segment stent struts in IVUS frames | Deep cascade learning | Using the DL algorithm, a reduced risk of ambiguities and false-positive predictions was observed for segmenting stents. |
| Blanco PJ, et al. 62 | 2022 | To determine the accuracy of a DL algorithm for automatic segmentation in IVUS images. | Multi-frame convolutional neural network, Gaussian process | The proposed DL approach provides accurate segmentations, which facilitates its implementation in clinical routine by mitigating the costs involved in the manual management of IVUS datasets. |
| Arora P, et al. 63 | 2023 | To use a DL algorithm to identify the extent of vascular calcification in IVUS images | AlexNet, GoogLeNet, SqueezeNet | DL algorithm identify the extension of vascular calcification with high accuracy. |
DL: deep learning; FFR: fractional flow reserve; IVUS: intravascular ultrasound; ML: machine learning; MPVI: morphological plaque vulnerability index; NIRS: near infrared spectroscopy; OCT: optical coherence tomography; SDH: stochastic driven histology; TCFA: thin-cap fibroatheroma; VH: virtual histology
Prediction of stent under-expansion using AI algorithms
Despite substantial improvements in interventional procedures, stent design, drugs and polymers, and the adoption of therapeutic strategies, acute stent thrombosis and in-stent restenosis remain critical issues. It can be difficult to fully expand a coronary artery stent in a heavily calcified coronary artery lesion. Careful evaluation of these risks of under-expansions before the intervention will aid treatment planning. AI-based systems play a crucial role in obtaining an automated and reproducible prediction since morphological characterization of atherosclerotic plaque and prediction of the risk of stent underexpansion are often challenging procedures.
IVUS is one of the most commonly intravascular procedures used to optimize stent implantation and has been associated with a decrease in adverse clinical events during follow-up. 2,3,4 However, IVUS has certain limitations, as determining the minimum stent area (MSA) in a single cross-section does not fully reflect the stent status along the entire vessel length, and there are no guidelines to predict the postprocedural MSA and the degree of stent expansion. In this context, CNN-based AI algorithms can automatically and adaptively calculate the probability of under-expansion after implantation. One study has developed an IVUS-based AI algorithm to predict the post-stenting stent area and the probability of under-expansion. 58 A dataset of pre-procedural and post-stenting IVUS images was obtained and was subsequently divided into a training set and a validation set for the algorithm. A DL CNN-based system was used to create a regression model to predict post-stenting stent area. XGBoost models for binary classification were developed to predict poststenting stent under-expansion (defined as an MSA < 5.5 mm2). MSA predicted by the pre-procedural IVUS-based regression model significantly correlated with those measured on post-stenting IVUS (r = 0.802; p < 0.001), and an accuracy of 94% was obtained to predict stent under-expansion (AUC = 0.94). 58 (Table 3)
Moderate to severe coronary artery calcification is a strong predictor of MACE after PCI. 64 This may be due to stent under-expansion caused by severely calcified plaque and inadequate lesion preparation prior to implantation. In this context, OCT-guided stent implantation provides a thorough assessment of coronary calcification and stent deployment characteristics, including aspects such as expansion, malapposition and stent edge dissection. 65 In addition, DL-based systems have been developed to optimize procedure duration and predict stent under-expansion using OCT. A retrospective single-center study analyzed OCT pullbacks with the goal of developing a DL algorithm to predict stent under-expansion before the procedure. 66 The DL algorithm exhibited re03kable discrimination ability to detect stent under-expansion, with an AUC of 0.853. This finding strongly supports the idea that AI systems may represent an extremely valuable addition to intravascular imaging methods, optimizing PCI. 66 In this context, we currently count with OCT imaging with AI-driven information (Ultreon™ 1.0 Software, Abbott). This software automatically detects the severity of coronary artery calcium, determines external elastic lamina and measures the vessel diameter, which not only increases the accuracy of real-time stenting, but also simplifies the interpretation of the acquired images, reducing interobserver variability 67.
Use of AI for segmentation and lumen area dimensions
In the assessment before PCI, it is crucial to accurately measure the vascular dimensions to select the adequate stent. Determining the length of the atherosclerotic plaque is crucial to avoid geographic miss during implantation. Additionally, knowing the lumen and vessel diameter is crucial in selecting the appropriate stent for implantation. One of the most arduous tasks when analyzing IVUS datasets is the segmentation of the lumen boundary and external elastic lamina, for which an expert has to manually outline them. This process is performed in the transversal axis and longitudinal view and usually results in large intra and interobserver variability. Additionally, artifacts are often present in IVUS images throughout the longitudinal view, and can lead to errors in lumen segmentation. To correct these errors, electrocardiogram-gating is necessary. Since this process is crucial for accurate data interpretation, AI algorithms are presented as an accurate and consistent alternative for selecting the correct frames. One study proposed an automated workflow to segment lumen boundaries in IVUS datasets using a DL approach and multi-frame (MF) CNN. The dataset consisted of IVUS pullbacks. After an automated gating, AI identified end-diastolic frames to avoid longitudinal artifacts and performed automated lumen segmentation using the MF-CNN algorithm. The correlation between the actual lumen and that obtained by the DL algorithm was high (r = 0.99) 55) (55 (Table 3). This DL algorithm has been extended for lumen and external elastic lamina segmentation with excellent results. 62 In turn, these results were replicated in other studies, such as a post-hoc, cross-sectional analysis of the IBIS-4 study 68, which evaluated the performance of a DL algorithm based on a MFCNN for automated lumen and vessel contour segmentation. 59 This study showed that, compared to the determination of vessel structures by human readers, the DL algorithm showed a strong correlation with those of the Core Lab. This offers the advantage of eliminating human inter and intraobserver variability, improving accuracy in lumen contour segmentation, and significantly reducing the time required to complete the analysis (average human time spent per pullback = 47 minutes vs. ML = 1 minute). 59
Utility of AI to optimize stent implantation
After PCI, it is essential to optimize stent placement to reduce the likelihood of adverse clinical events during follow-up. Intravascular imaging guidance using IVUS or OCT provides clear advantages over conventional angiographic guidance in terms of optimization. However, determining the parameters to be corrected in complex PCI procedures can be a challenging task. In this context, three-dimensional (3D) reconstruction of implanted stents using intravascular imaging methods is useful for addressing implantation issues. Of note, as manual reconstruction is a complicated process, AI algorithms can be a valuable tool. The introduction of DL algorithms has revolutionized automatic stent 3D reconstruction visualized by intravascular imaging methods, enabling reliable and real-time analysis during the intervention. 61 OCT and IVUS have different image styles, share the same anatomic structures, and can be aligned by cross-modal translation. In this context, a study explored the feasibility of using OCT and IVUS to assist each other in DL-based automatic 3D reconstruction of the stents implanted during PCI. The study found that the DL algorithm performs exceptionally well in generating 3D reconstructions of implanted stents, regardless of whether conventional IVUS or high-definition IVUS were used for optimization. This information can be valuable when analyzing potential improvements and optimizations in the implantation procedure. 45 (Table 2).
CHALLENGES AND FUTURE PERSPECTIVES
Considering that AI is a data-driven science, the lack of standardization in collecting and storing medical data may negatively influence the interoperability of AI systems in the medical field. Data heterogeneity hinders the effective integration of AI algorithms in different clinical settings and the communication of information between healthcare systems.
Furthermore, the performance of AI algorithms in clinical practice depends heavily on the representativeness of the training data compared to the data encountered in daily practice. In some cases, the data collected for developing AI algorithms may not be representative of the population it serves. leading to poor performance. In this sense, external validation and field testing are essential to determine the level of confidence of AI algorithms. As artificial AI is already being integrated into clinical workflows, it is essential to demonstrate its value in patient care, support investment in these new algorithms, and encourage the adoption of new reimbursement or payment models. To achieve this on a large scale, it is essential to conduct cost-effectiveness studies of these emerging technologies.
CONCLUSIONS
When combined with intravascular imaging methods such as IVUS or OCT, AI systems significantly optimize stent implantation through a fully automated framework that identifies vascular structures and guides correct stent positioning. This facilitates the accurate selection of the stent to be implanted and the need for stent correction techniques. Additionally, it reduces both the total procedure time and interobserver variability. This could promote greater adoption of intravascular imaging techniques and ultimately reduce the incidence of adverse clinical events during follow-up.
REFERENCIAS BIBLIOGRÁFICAS
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation [Internet]. 2021 Feb 23.[cited 2023.];143(8):E254-743. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33501848/ [ Links ]
2. Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JAMA [Internet]. 2015 Nov 24.[cited 2023.];314(20):2155-63. Available from: Available from: https://jamanetwork.com/journals/jama/fullarticle/2469205 [ Links ]
3. Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation: The ULTIMATE Trial. J Am Coll Cardiol [Internet]. 2018 Dec 18.[cited 2023.];72(24):3126-37. Available from: Available from: https://www.jacc.org/doi/10.1016/j.jacc.2018.09.013 [ Links ]
4. Lee JM, Choi KH, Song Y Bin, Lee J-Y, Lee S-J, Lee SY, et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N Engl J Med [Internet]. 2023 05 4 [cited 2023 Oct 1.];388(18):1668-79. Available from: Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2216607 [ Links ]
5. Holm NR, Andreasen LN, Neghabat O, Laanmets P, Kumsars I, Bennett J, et al. 10 or Angiography Guidance for PCI in Complex Bifurcation Lesions. N Engl J Med [Internet]. 2023 Aug 26.[cited 2023.]; Available from: Available from: https://www.nejm.org/doi/full/10.1056/NEJMoa2307770 [ Links ]
6. Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism [Internet]. 2017 Apr 1 [cited 2023.];69S:S36-40. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/28126242/ [ Links ]
7. McCarthyJohn, L. M, RochesterNathaniel, E. S. A Proposal for the Dartmouth Summer Research Project on Artificial Intelligence. AI Mag [Internet]. 2006 Dec 1 [cited 2023.]; Available from: Available from: https://dl.acm.org/doi/10.1609/aimag.v27i4.1904 [ Links ]
8. Lindsay RK, Buchanan B, Feigenbaum E, Lederberg J. Applications of Artificial Intelligence for Organic Chemistry: The DENDRAL Project. 1980; [ Links ]
9. Johnson KW, Torres Soto J, Glicksberg BS, Shameer K, Miotto R, Ali M, et al. Artificial Intelligence in Cardiology. J Am Coll Cardiol [Internet]. 2018.[cited 2023.];71(23):2668-79. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/29880128/ [ Links ]
10. Zhang Z, Sejdić E. Radiological images and machine learning: trends, perspectives, and prospects. Comput Biol Med [Internet]. 2019 May 1 [cited 2023.];108:354 Available from: /pmc/articles/PMC6531364/ [ Links ]
11. Gessert N, Lutz M, Heyder M, Latus S, Leistner DM, Abdelwahed YS, et al. Automatic Plaque Detection in IV10 Pullbacks Using Convolutional Neural Networks. IEEE Trans Med Imaging. 2019 Feb 1;38(2):426-34. [ Links ]
12. Abdolmanafi A, Duong L, Dahdah N, Cheriet F. Deep feature learning for automatic tissue classification of coronary artery using optical coherence tomography. Biomed Opt Express [Internet]. 2017 Feb 1 [cited 2023.];8(2):1203. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/28271012/ [ Links ]
13. Li L, Jia T. Optical Coherence Tomography Vulnerable Plaque Segmentation Based on Deep Residual U-Net. Rev Cardiovasc Med [Internet]. 2019 Sep 30.[cited 2023.];20(3):171-7. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/31601091/ [ Links ]
14. Nair A, Kuban BD, Obuchowski N, Geoffrey Vince D. Assessing spectral algorithms to predict atherosclerotic plaque composition with normalized and raw intravascular ultrasound data. Ultrasound Med Biol [Internet]. 2001 [cited 2023.];27(10):1319-31. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/11731045/ [ Links ]
15. Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation [Internet]. 2002 Oct 22.[cited 2023.];106(17):2200-6. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/12390948/ [ Links ]
16. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science [Internet]. 1991 [cited 2023.];254(5035):1178-81. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/1957169/ [ Links ]
17. Pighi M, Gratta A, marin F, Bellamoli M, Lunardi M, Fezzi S, et al. “Cardiac allograft vasculopathy: Pathogenesis, diagnosis and therapy.” Transplant Rev (Orlando) [Internet]. 2020 Oct 1 [cited 2023.];34(4). Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/32739137/ [ Links ]
18. Chu M, Jia H, Gutiérrez-Chico JL, Maehara A, Ali ZA, Zeng X, et al. Artificial intelligence and optical coherence tomography for the automatic characterisation of human atherosclerotic plaques. Euro- Intervention. 2021 May 1;17(1):41-50. [ Links ]
19. Hong H, Jia H, Zeng M, Gutiérrez-Chico JL, Wang Y, Zeng X, et al. Risk Stratification in Acute Coronary Syndrome by Comprehensive Morphofunctional Assessment With Optical Coherence Tomography. JACC Asia. 2022 Aug 1).;2(4):460-72. [ Links ]
20. Athanasiou LS, Exarchos TP, Naka KK, Michalis LK, Prati F, Fotiadis DI. Atherosclerotic plaque characterization in Optical Coherence Tomography images. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf [Internet]. 2011 [cited 2024.];2011:4485-8 Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/22255335/ [ Links ]
21. Wang Z, Chamie D, Bezerra HG, Yamamoto H, Ka11sky J, Wilson DL, et al. Volumetric quantification of fibrous caps using intravascular optical coherence tomography. Biomed Opt Express , Vol 3, Issue 6, pp 1413-1426 [Internet]. 2012 Jun 1.[cited 2024.];3(6):1413-26. Available from: Available from: https://opg.optica.org/viewmedia.cfm?uri=boe-3-6-1413&seq=0&html=true [ Links ]
22. Ughi GJ, Adriaenssens T, Sinnaeve P, Desmet W, D’hooge J. Automated tissue characterization of in vivo atherosclerotic plaques by intravascular optical coherence tomography images. Biomed Opt Express [Internet]. 2013 Jul 1.[cited 2024.];4(7):1014. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/23847728/ [ Links ]
23. Xu M, Cheng J, Wong DWK, Taruya A, Tanaka A, Liu J. Automatic atherosclerotic heart disease detection in intracoronary optical coherence tomography images. 2014 36th Annu Int Conf IEEE Eng Med Biol Soc EMBC 2014. 2014 11 2;174-7. [ Links ]
24. Wang Z, Jia H, Tian J, Soeda T, Vergallo R, Minami Y, et al. Computer-aided image analysis algorithm to enhance in vivo diagnosis of plaque erosion by intravascular optical coherence tomography. Circ Cardiovasc Imaging [Internet]. 2014 Sep 1.[cited 2024.];7(5):805-10. Available from: Available from: https://www.ahajournals.org/doi/abs/10.1161/CIRCIMAGING.114.002084 [ Links ]
25. Shalev R, Bezerra HG, Ray S, Prabhu D, Wilson DL. Classification of calcium in intravascular 10 images for the purpose of intervention planning. Doi: 101117/122216315 [Internet]. 2016 Mar 18.[cited 2024.];9786:50-62 Available from: Available from: https://www. spiedigitallibrary.org/conference-proceedings-of-spie/9786/978605/Classification-of-calcium-in-intravascular-10-images-for-the-purpose/ 10.1117/12.2216315.full [ Links ]
26. Rico-Jimenez JJ, Campos-Delgado DU, Villiger M, Otsuka K, Bouma BE, Jo JA. Automatic classification of atherosclerotic plaques imaged with intravascular 10. Biomed Opt Express [Internet]. 2016 Oct 1.[cited 2024.];7(10):4069. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/27867716/ [ Links ]
27. Xu M, Cheng J, Li A, Lee JA, Wing D, Wong K, et al. Fibroatheroma Identification in Intravascular Optical Coherence Tomography Images using Deep Features. [ Links ]
28. Shi P, Xin J, Liu S, Deng Y, Zheng N. Vulnerable Plaque Recognition Based on Attention Model with Deep Convolutional Neural Network. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Int Conf [Internet]. 2018 Oct 26.[cited 2024.];2018:834-7 Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/30440521/ [ Links ]
29. Guo X, Tang D, Molony D, Yang C, Samady H, Zheng J, et al. A Machine Learning-Based Method for Intracoronary 10 Segmentation and Vulnerable Coronary Plaque Cap Thickness Quantification. https://doi.org/101142/S0219876218420082 [Internet]. 2019.[cited 2024.];16(3). Available from: Available from: https://www.worldscientific.com/worldscinet/ijcm [ Links ]
30. Kolluru C, Prabhu D, Gharaibeh Y, Bezerra H, Guagliumi G, Wilson D. Deep neural networks for A-line-based plaque classification in coronary intravascular optical coherence tomography images. https://doi.org/101117/1JMI54044504 [Internet]. 2018 Dec 3.[cited 2024.];5(4):044504. Available from: Available from: https://www.spiedigitallibrary.org/journals/journal-of-medical-imaging/volume-5/issue-4/044504/Deep-neural-networks-for-A-line-based-plaque-classification-in/10.1117/1.JMI.5.4.044504.full [ Links ]
31. He S, Zheng J, Maehara A, Mintz G, Tang D, Anastasio M, et al. Convolutional neural network based automatic plaque characterization from intracoronary optical coherence tomography images. 2018 Jul 10.[cited 2024.];107. Available from: Available from: http://arxiv.org/abs/1807.03613 [ Links ]
32. Lee J, Prabhu D, Kolluru C, Gharaibeh Y, Zimin VN, Bezerra HG, et al. Automated plaque characterization using deep learning on coronary intravascular optical coherence tomographic images. Biomed Opt Express [Internet]. 2019 Dec 1.[cited 2024.];10(12):6497. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/31853413/ [ Links ]
33. Prabhu DS, Bezerra HG, Kolluru C, Gharaibeh Y, Mehanna E, Wu H, et al. Automated A-line coronary plaque classification of intravascular optical coherence tomography images using handcrafted features and large datasets. J Biomed Opt [Internet]. 2019 Oct 4.[cited 2024.];24(10):1. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/31586357/ [ Links ]
34. Liu R, Zhang Y, Zheng Y, Liu Y, Zhao Y, Yi L. Automated Detection of Vulnerable Plaque for Intravascular Optical Coherence Tomography Images. Cardiovasc Eng Technol [Internet]. 2019 Dec 1.[cited 2024.];10(4):590-603. Available from: Available from: https://link.springer.com/article/10.1007/s13239-019-00425-2 [ Links ]
35. Johnson KW, Glicksberg BS, Shameer K, Vengrenyuk Y, Krittanawong C, Russak AJ, et al. A transcriptomic model to predict increase in fibrous cap thickness in response to high-dose statin treatment: Validation by serial intracoronary 10 imaging. EBio- Medicine [Internet]. 2019 Jun 1.[cited 2024.];44:41-9 Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/31126891/ [ Links ]
36. Baruah V, Zahedivash A, Hoyt T, McElroy A, Vela D, Buja LM, et al. Automated Coronary Plaque Characterization With Intravascular Optical Coherence Tomography and Smart-Algorithm Approach: Virtual Histology 10. JACC Cardiovasc Imaging. 2020 Aug 1).;13(8):1848-50. [ Links ]
37. Lee J, Prabhu D, Kolluru C, Gharaibeh Y, Zimin VN, Dallan LAP, et al. Fully automated plaque characterization in intravascular 10 images using hybrid convolutional and lumen morphology features. Sci Rep [Internet]. 2020 Dec 1.[cited 2024.];10(1). Available from: /pmc/articles/PMC7018759/ [ Links ]
38. Lee J, Gharaibeh Y, Kolluru C, Zimin VN, Dallan LAP, Kim JN, et al. Segmentation of Coronary Calcified Plaque in Intravascular 10 Images Using a Two-Step Deep Learning Approach. IEEE access Pract In11 open Solut [Internet]. 2020 [cited 2024.];8:225581-93 Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33598377/ [ Links ]
39. Cha JJ, Son TD, Ha J, Kim JS, Hong SJ, Ahn CM, et al. Optical coherence tomography-based machine learning for predicting fractional flow reserve in intermediate coronary stenosis: a feasibility study. Sci Rep [Internet]. 2020 Dec 1.[cited 2024.];10(1). Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33235309/ [ Links ]
40. Balaji A, Kelsey LJ, Majeed K, Schultz CJ, Doyle BJ. Coronary artery segmentation from intravascular optical coherence tomography using deep capsules. Artif Intell Med [Internet]. 2021 Jun 1.[cited 2024.];116:102072 Available from: Available from: https://research-repository.uwa.edu.au/en/publications/coronary-artery-segmentation-fromintravascular-optical-coherence [ Links ]
41. Yin Y, He C, Xu B, Li Z. Coronary Plaque Characterization From Optical Coherence Tomography Imaging With a Two-Pathway Cascade Convolutional Neural Network Architecture. Front Cardiovasc Med. 2021 Jun 16).;8:670502 [ Links ]
42. Li C, Jia H, Tian J, He C, Lu F, Li K, et al. Comprehensive Assessment of Coronary Calcification in Intravascular 10 Using a Spatial-Temporal Encoder-12oder Network. IEEE Trans Med Imaging . 2022 Apr 1).;41(4):857-68. [ Links ]
43. Yang G, Yang G, Mehanna E, Mehanna E, Li C, Zhu H, et al. Stent detection with very thick tissue coverage in intravascular 10. Biomed Opt Express , Vol 12, Issue 12, pp 7500-7516 [Internet]. 2021 Nov 21.[cited 2024.];12(12):7500-16. Available from: Available from: https://opg.optica.org/viewmedia.cfm?uri=boe-12-12-7500&seq=0&html=true [ Links ]
44. Sun H, Sun H, Zhao C, Zhao C, Zhao C, Qin Y, et al. In vivo detection of plaque erosion by intravascular optical coherence tomography using artificial intelligence. Biomed Opt Express , Vol 13, Issue 7, pp 3922-3938 [Internet]. 2022 Jun 1.[cited 2024.];13(7):3922-38. Available from: Available from: https://opg.optica.org/viewmedia.cfm?uri=boe-13-7-3922&seq=0&html=true [ Links ]
45. Wu P, Qiao Y, Chu M, Zhang S, Bai J, Gutierrez-Chico JL, et al. Reciprocal assistance of intravascular imaging in three-dimensional stent reconstruction: Using cross-modal translation based on disentanglement representation. Comput Med Imaging Graph [Internet]. 2023 Mar 1.[cited 2023.];104. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/36586195/ [ Links ]
46. Hoffmann U, Massaro JM, D’Agostino RB, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc [Internet]. 2016 Feb 1.[cited 2023.];5(2). Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/26903006/ [ Links ]
47. Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Chuang YC, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation [Internet]. 1995 Apr 1.[cited 2023.];91(7):1959-65. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/7895353/ [ Links ]
48. Neleman T, Liu S, Tovar Forero MN, Hartman EMJ, Ligthart JMR, Witberg KT, et al. The Prognostic Value of a Validated and Automated Intravascular Ultrasound-Derived Calcium Score. J Cardiovasc Transl Res [Internet]. 2021 Oct 1.[cited 2024.];14(5):992-1000. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33624259/ [ Links ]
49. Agatston AS, 01owitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol [Internet]. 1990 Mar 15.[cited 2023.];15(4):827-32. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/2407762/ [ Links ]
50. Sheet D, Karamalis A, Eslami A, Noël P, Chatterjee J, Ray AK, et al. Joint learning of ultrasonic backscattering statistical physics and signal confidence primal for characterizing atherosclerotic plaques using intravascular ultrasound. Med Image Anal [Internet]. 2014 Feb [cited 2024.];18(1):103-17. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/24184434/ [ Links ]
51. Kim GY, Lee JH, Hwang YN, Kim SM. A 11el intensity-based multi-level classification approach for coronary plaque characterization in intravascular ultrasound images. Biomed Eng Online [Internet]. 2018 Nov 6.[cited 2024.];17(Suppl 2). Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/30396344/ [ Links ]
52. Bae Y, Kang SJ, Kim G, Lee JG, Min HS, Cho H, et al. Prediction of coronary thin-cap fibroatheroma by intravascular ultrasoundbased machine learning. Atherosclerosis [Internet]. 2019 Sep 1.[cited 2024.];288:168-74 Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/31130215/ [ Links ]
53. Jun TJ, Kang SJ, Lee JG, Kweon J, Na W, Kang D, et al. Automated detection of vulnerable plaque in intravascular ultrasound images. Med Biol Eng Comput [Internet]. 2019 Apr 11.[cited 2024.];57(4):863-76. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/30426362/ [ Links ]
54. Wang L, Tang D, Maehara A, Wu Z, Yang C, Muccigrosso D, et al. Using intravascular ultrasound image-based fluid-structure interaction models and machine learning methods to predict human coronary plaque vulnerability change. Comput Methods Biomech Biomed Engin [Internet]. 2020 [cited 2024 Feb 27.];23(15):1267-76. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/32696674/ [ Links ]
55. Ziemer PGP, Bulant CA, Orlando JI, Maso Talou GD, Álvarez LAM, Guedes Bezerra C, et al. Automated lumen segmentation using multi-frame convolutional neural networks in intravascular ultrasound datasets. Eur Hear journal Digit Heal [Internet]. 2020.[cited 2023 Oct 2.];1(1):75-82. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/36713961/ [ Links ]
56. Lee JG, Ko J, Hae H, Kang SJ, Kang DY, Lee PH, et al. Intravascular ultrasound-based machine learning for predicting fractional flow reserve in intermediate coronary artery lesions. Atherosclerosis . 2020 Jan 1).;292:171-7. [ Links ]
57. Cho H, Kang SJ, Min HS, Lee JG, Kim WJ, Kang SH, et al. Intravascular ultrasound-based deep learning for plaque characterization in coronary artery disease. Atherosclerosis [Internet]. 2021 May 1.[cited 2024.];324:69-75. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33831671/ [ Links ]
58. Min HS, Ryu D, Kang SJ, Lee JG, Yoo JH, Cho H, et al. Prediction of Coronary Stent Underexpansion by Pre-Procedural Intravascular Ultrasound-Based Deep Learning. JACC Cardiovasc Interv [Internet]. 2021 May 10.[cited 2024.];14(9):1021-9. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/33865741/ [ Links ]
59. Bass RD, Garcia-Garcia HM, Sanz-Sánchez J, Ziemer PGP, Bulant CA, Kuku KK, et al. Human vs. machine vs. core lab for the assessment of coronary atherosclerosis with lumen and vessel contour segmentation with intravascular ultrasound. Int J Cardiovasc Imaging [Internet]. 2022 Jul 1.[cited 2024.];38(7):1431-9. Available from: Available from: https://www.x-mol.net/paper/article/1516497141295915008 [ Links ]
60. Bajaj R, Eggermont J, Grainger SJ, Räber L, Parasa R, Khan AHA, et al. Machine learning for atherosclerotic tissue component classification in combined near-infrared spectroscopy intravascular ultrasound imaging: Validation against histology. Atherosclerosis . 2022 Mar 1).;345:15-25. [ Links ]
61. Wissel T, Riedl KA, Schaefers K, Nickisch H, Brunner FJ, Schnellbaecher ND, et al. Cascaded learning in intravascular ultrasound: coronary stent delineation in manual pullbacks. https://doi.org/101117/1JMI92025001 [Internet]. 2022 Mar 28.[cited 2023.];9(2):025001. Available from: Available from: https://www.spiedigitallibrary.org/journals/journal-of-medical-imaging/volume-9/issue-2/025001/Cascaded-learning-in-intravascular-ultrasound--coronary-stent-delineation-in/10.1117/1.JMI.9.2.025001.full [ Links ]
62. Blanco PJ, Ziemer PGP, Bulant CA, Ueki Y, Bass R, Räber L, et al. Fully automated lumen and vessel contour segmentation in intravascular ultrasound datasets. Med Image Anal [Internet]. 2022 Jan 1.[cited 2023.];75. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/34670148/ [ Links ]
63. Arora P, Singh P, Girdhar A, Vijayvergiya R. Calcification Detection in Intravascular Ultrasound (IVUS) Images Using Transfer Learning Based MultiSVM model. https://doi.org/101177/01617346231164574 [Internet]. 2023 Apr 13.[cited 2024.]; Available from: Available from: https://journals.sagepub.com/doi/10.1177/01617346231164574 [ Links ]
64. Généreux P, Madhavan M V., Mintz GS, Maehara A, Palmerini T, Lasalle L, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization. J Am Coll Cardiol [Internet]. 2014 May 13.[cited 2023.];63(18):1845-54.) Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/24561145/ [ Links ]
65. Fujino A, Mintz GS, Matsumura M, Lee T, Kim SY, Hoshino M, et al. A new optical coherence tomography-based calcium scoring system to predict stent underexpansion. EuroIntervention [Internet]. 2018 Apr 1.[cited 2023 Oct 5.];13(18):e2182-9. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/29400655/ [ Links ]
66. Gharaibeh Y, Lee J, Zimin VN, Kolluru C, Dallan LAP, Pereira GTR, et al. Prediction of stent under-expansion in calcified coronary arteries using machine-learning on intravascular optical coherence tomography. 2022 May 16.[cited 2023.]; Available from: Available from: https://arxiv.org/abs/2205.10354v1 [ Links ]
67. Bartuś S, Siłka W, Kasprzycki K, Sabatowski K, Malinowski KP, Rzeszutko Ł, et al. Experience with Optical Coherence Tomography Enhanced by a 11el Software (UltreonTM 1.0 Software)-The First One Hundred Cases. Medicina (Kaunas) [Internet]. 2022 Sep 1.[cited 2023.];58(9). Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/36143904/ [ Links ]
68. Räber L, Taniwaki M, Z08g S, Kelbãk H, Roffi M, Holmvang L, et al. Effect of high-intensity statin therapy on atherosclerosis in non-infarct-related coronary arteries (IBIS-4): a serial intravascular ultrasonography study. Eur Heart J [Internet]. 2015 Feb 21.[cited 2023.];36(8):490-500. Available from: Available from: https://pubmed.ncbi.nlm.nih.gov/25182248/ [ Links ]
Received: November 23, 2023; Accepted: January 19, 2024











 texto em
texto em