Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cardiología
versão On-line ISSN 1850-3748
Rev. argent. cardiol. vol.92 no.1 Ciudad Autónoma de Buenos Aires mar. 2024 Epub 28-Fev-2024
http://dx.doi.org/10.7775/rac.es.v92.i1.20729
ORIGINAL ARTICLE
Should We Continue to Consider Extent of Ischemic Myocardium in Chronic Coronary Syndromes?
1 Nuclear Cardiology Division , Instituto Cardiovascular de Buenos Aires, Buenos Aires, Argentina
2 Nuclear Cardiology Division , Hospital JM Ramos Mejía, Buenos Aires, Argentina
3 Nuclear Cardiology Division , Hospital Cosme Argerich, Buenos Aires, Argentina
4 Cardio Imaging, TCba Salguero Buenos Aires, Argentina
In the last years, new scientific evidence related to chronic coronary syndromes (CCS) has led to reconsider the diagnostic and therapeutic recommendations that historically guided our medical practice.
However, it is important to point out that the disseminated information lacks a precise critical analysis, with the risk of incorporating new algorithms in clinical practice that might not be completely applicable to our population.
The present review analyses the available scientific CCS literature to establish whether the extent of ischemic myocardium has no real clinical and prognostic significance as reported in some publications.
Key words: Chronic coronary syndromes; Ischem; - Revascularization
INTRODUCTION
There are currently six clinical scenarios of chronic coronary syndromes (CCS): 1- Patients with stable symptoms (angina or dyspnea); 2- Patients with de novo heart failure (HF) or left ventricular disfunction and suspicion of coronary artery disease (CAD); 3- Symptomatic or asymptomatic patients with < 1 year acute coronary syndrome (ACS) or recent revascularization; 4- Symptomatic or asymptomatic patients with >1 year of revascularization; 5- Patients with vasospasm or microvascular disease; and 6- Asymptomatic patients with CAD findings. 1
For almost two decades, the therapeutic conduct of CCS was ruled by the extent of myocardial ischemia found in functional tests, as recommended by the last European Society of Cardiology and the Argentine Society of Cardiology guidelines. 1),(2
This was mainly due to the contributions of the study performed by Hachamovitch et al., published in 2003, which demonstrated that early revascularization compared with optimal medical treatment (OMT) presented less risk of short-term cardiovascular mortality in patients without known CAD and moderate to severe inducible ischemia (>10%) assessed by single photon emission computed tomography (SPECT). 3),(4
Years later, the same author found again an interaction between inducible ischemia, the established treatment and all-cause mortality. He identified a benefit of greater survival with revascularization over OMT in the context of extensive ischemia, both in patients without known CAD, as among those with revascularization, but no previous infarct. 5
The ISCHEMIA study
Recently, the ISCHEMIA trial cast doubt on this concept, since no benefit was found with an early invasive strategy in a median follow-up of 3.2 years. 6
However, some key points of this study deserve our special attention in order to perform a critical analysis, and not fall in interpretation errors and incorrectly extrapolate their results to different populations of chronic coronary patients.
As recalled, in the ISCHEMIA study, 5179 patients with moderate or severe ischemia were randomly assigned to an initial invasive strategy (angiography and revascularization whenever possible) and medical treatment vs. an initial conservative strategy of medical therapy alone and angiography only in case of medical treatment failure. The primary endpoint was a composite of cardiovascular death, myocardial infarction (AMI) or hospitalization for unstable angina, heart failure or reanimated cardiac arrest. The secondary endpoint was cardiovascular death or AMI. 6 The study only included patients comprised in groups 1 and 4 of CCS, and did not include patients with reduced left ventricular ejection fraction (LVEF) (median value of 60%), nor those with hospitalization for heart failure or symptoms exacerbation in the last 6 months. Only patients in NYHA functional class I-II were randomized. In addition, at the time of recruitment, they did not have to present left main coronary artery disease ≥50%, refractory symptoms to OMT, nor unfavorable anatomy for revascularization. 7
The Seattle angina scale score was 80±20 in the invasive strategy group and 82±19 in the OMT group, indicating that most patients were asymptomatic or slightly symptomatic at inclusion time. 8 Functional tests with imaging studies were employed in 75.5% of patients, SPECT being the most used modality (49.6%) followed by stress echocardiography (20.9%) and cardiac magnetic resonance (5%). Regarding severity, 41% of participants presented moderate ischemia defined by an extension < 10%, or by an “enlarged” definition which combined 5% ischemic myocardium plus a clinical history of angina, associated with a theoretical maximum heart rate ≤75% and less than 7 METS in the ergometric test. Severe ischemia (>10%) was present in 45% of cases; however, the prevalence and analysis of more extended ischemia (15% or 20%) is unknown. 7
It must be considered that as the decision to include a patient depended on the treating physician, some inclusion bias could have occurred, as for example patients with more extended severe ischemia, probably associated with higher risk of adverse events, who wouldn’t had been randomized but referred directly to a revascularization procedure (Figure 1).
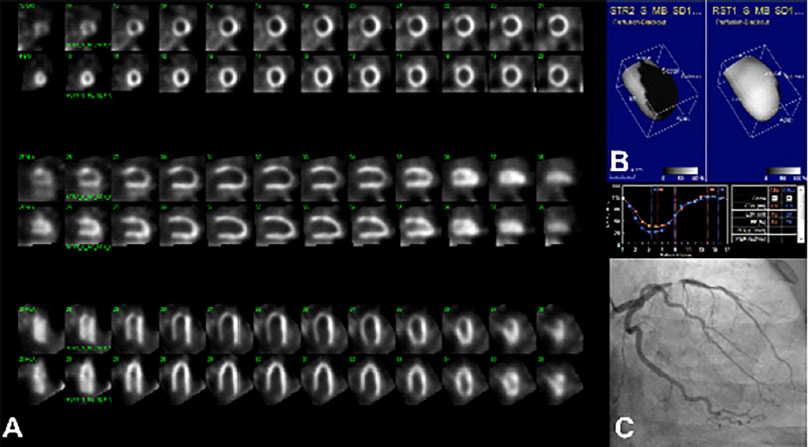
Fig. 1 Would you have enrolled this patient in the ISCHEMIA study? 51-year-old male patient, with suspected coronary artery disease due to stable angina in FC II-III. A. Rest-stress myocardial SPECT evidencing severe myocardial ischemia (35% in the left anterior descending (LAD) artery territory. B. Surface mapping showing the reversible perfusion defect and preserved left ventricular ejection fraction at rest and after exercise stress test. C. Coronary angiography showing subtotal lesion in the mid third of the LAD artery.
The slow recruitment resulted in a reduced sample size (from 8000 planned patients to 5179 effectively randomized) and to “force” the inclusion of some patients. In 14% of cases incorporated as “moderate ischemia”, the central laboratory did not validate the extension. Twenty-five percent of recruited participants were included for presenting a positive ergometric test associated with an anatomical study, coronary angiography (CA) or computed tomography angiography (CTA) that demonstrated ≥70% disease in the proximal or mid third left anterior descending artery or right coronary artery and/or proximal third of the circumflex artery. 7
This last inclusion criteria, which incorporates anatomical information without knowledge of the ischemic extent, reflects the heterogeneity of the population.
Is the anatomy enough?
Anatomical knowledge through CTA is very useful due to its high negative predictive value (NPV); however, it has a moderate positive predictive value (PPV), close to 60-70%, if compared with CA or stress functional tests with imaging studies. This limitation is due to the difficulty in evaluating moderate lesions, the presence of coronary calcium and the interobserver variability.
To settle this inconvenience, various studies postulated as alternative the addition of the non-invasive fractional flow reserve (FFR) measurement to CTA. Nevertheless, a recent publication analyzed the usefulness and efficacy of this measurement through a multicenter audit developed in England, and the results showed that, even adding FFR, CTA PPV is still low (near 50%) and potentially more expensive than the conventional strategies that use functional tests. 9),(10
The PROMISE study included 10 003 symptomatic patients with suspected CAD and an intermediate pretest probability of 53.3%, which were randomly assigned to an initial strategy of functional tests (SPECT, stress echocardiogram and ergometry) vs. a CTA anatomical study. The initial strategy with CTA (versus functional tests) did not improve the clinical results during a median 2-year follow-up. In both arms the rate of primary events (death, AMI, hospitalization for unstable angina or severe complication of the procedure) was similar: 3.3% vs. 3%. However, a larger number of patients assigned to the CTA group underwent cardiac catheterization within 90 days after randomization (12.2% vs. 8.1% in the functional tests arm) and a greater percentage of patients in the CTA group were revascularized (6.2% vs. 3.2%). 11
Going back to the ISCHEMIA trial, it should be recalled that this was not designed to evaluate the clinical value of functional tests (there was no control group without ischemic evoking test or with a negative functional test), but to assess two treatment strategies. 12
In our setting, it would be scarcely feasible and highly costly, to initiate the study of all asymptomatic or slightly symptomatic patients with suspected CAD by CTA or CA. In addition, it could needlessly and prematurely lead to perform revascularization procedures without a clear prognostic benefit, as shown by several clinical trials. 13),(14
Adding evidence
In the years after the publication of the ISCHEMIA study other studies, though with lower dissemination, were nonetheless important to complement our knowledge at the time of deciding patient management in CCS. Such is the case of the study published by Rozanski et al., who analyzed the relationship between stress-induced myocardial ischemia and all-cause mortality in patients with preserved vs. reduced LVEF. A total of 43 443 patients were evaluated with SPECT and with a long-term follow-up of 11.4 years. Patients with LVEF ≥45% and severe myocardial ischemia and those with LVEF < 45% and moderate or severe myocardial ischemia benefitted with early revascularization. It should be pointed out that the percentage of ischemic myocardium extent in this publication differed substantially from that considered in the ISCHEMIA study: up to 5% of reversible defect was not considered ischemia, from 5-9% mild ischemia, from 10-14% moderate ischemia and ≥15 % severe ischemia. 15
These results were similar to those reported in another retrospective trial of 16 029 patients evaluated by positron emission computed tomography (PET), where a significant interaction between ischemic extent and early revascularization was also demonstrated, so patients with larger ischemia had better survival if they underwent revascularization within 90 days after the myocardial perfusion test. 16
In this same line, the REFINE study retrospectively analyzed a population of 19 088 patients evaluated by SPECT, in whom early revascularization decreased the rate of major events (mortality, AMI, and unstable angina) in patients with ≥10.2% ischemia. 17
As prior history of these works, a subanalysis of the COURAGE study analyzed 621 patients with an ischemic extent of 14-17%, and in whom a certain benefit of revascularization was found. (p=0.03). However, as they were very few patients, the authors did not consider it among their conclusions, despite a statistically significant difference. 18
Although these reports emerged from retrospective analyses, lack of treatment intervention / control and the absence of a possible inclusion bias, has perhaps turned these patients to be more similar to those we find and treat in the “real world” of cardiological practice.
Recommendations, doubts and certainties
The CCS guideline recently published by the American Heart Association (AHA) establishes that in patients with known CAD who persist symptomatic despite OMT, it is recommended to perform a functional test to detect the presence and extent of ischemia (IB recommendation), to determine the risk of major events and guide the therapeutic conduct. 19
However, this document has generated some uncertainty among clinical cardiologists, as it also postulated guiding treatment according to anatomical findings, with the same recommendation level. Hence a new question arises: patients in whom the diagnosis is initiated by the anatomy, and severe proximal lesions are found in one or two vessels, ¿would they be amenable to receive OMT, or be revascularized without evidence that a functional involvement generating ischemia really exists?
If the diagnosis started by the anatomy, it should be considered that epicardial arteries are only 5% of the coronary tree and that anginal episodes and focal epicardial lesions do not always go hand in hand. Other pathophysiological mechanisms could generate an unbalance between myocardial oxygen offer and demand, unrelated to the atherosclerotic disease. 19),(20),(21
Currently, a growing number of patients with CCS present with microvascular angina. 22),(23),(24),(25 Although this is more frequent in patients without significant angiographic lesions, it can be present in patients with known CAD and in up to one third of revascularized patients that continue to manifest anginal episodes after revascularization. 26),(27
The ISCHEMIA study did not include participants with less than 12 months since the last revascularization. This group deserves a special mention, as a percentage of them can present stent or vascular graft stenosis, with no other abnormal finding than myocardial perfusion or regional wall motility. The detection of ischemia by functional tests in this group of still asymptomatic patients, worsens the prognosis. 28),(29
The evidence thus continues to be controversial, and is still not enough to confirm that the ischemic myocardium extent has lost the significance indicated more than 20 years ago. Each patient should be analyzed in his/her clinical context and socioeconomic environment. Functional and anatomical information should continue to be complementary and not necessarily excluding.
According to the present report, we consider that even today, knowing the presence and extent of ischemia in patients with CCS can modify their prognosis and treatment. 30
“The mind is like a parachute… It only works if it is open”, “We are all very ignorant. What happens is that not all of us ignore the same things”. Albert Einstein.
BIBLIOGRAFIA
1. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck- Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:407-77. https://doi.org/10.1161/10.1093/eurheartj/ehz425 [ Links ]
2. Gagliardi J, Cestari G, Llois S, Ferroni F, Meretta A, Ahuad Guerrero A. Consenso de Síndromes Coronarios Crónicos. Resumen de las Recomendaciones 2019. Rev Argent Cardiol 2020;88:1-74. [ Links ]
3. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-7. https://doi.org/10.1161/01.CIR.0000072790.23090.41 [ Links ]
4. Hachamovitch R, Rozanski A, Hayes SW, Thomson LE, Germano G, Friedman JD, et al. Predicting therapeutic benefit from myocar- REFERENCES dial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol. 2006;13:768-78. https://doi.org/10.1016/j.nuclcard.2006.08.017 [ Links ]
5. Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012-24. https://doi.org/10.1093/eurheartj/ehq500 [ Links ]
6. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. ISCHEMIA Research Group. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395-407. https://doi.org/10.1161/10.1056/NEJMoa1915922 [ Links ]
7. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. Supplementary Appendix. N Engl J Med 2020;382:1395-407. [ Links ]
8. Thomas M, Jones PG, Arnold SV, Spertus JA. Interpretation of the Seattle Angina Questionnaire as an Outcome Measure in Clinical Trials and Clinical Care: A Review.JAMA Cardiol. 2021;6:593-9. https://doi.org/10.1001/jamacardio.2020.7478 [ Links ]
9. Patel MR, Nørgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT: The ADVANCE Registry. JACC Cardiovasc Imaging. 2020;13:97-105. https://doi.org/10.1016/j.jcmg.2019.03.003 [ Links ]
10. Mittal TK, Hothi SS, Venugopal V, Taleyratne J, O'Brien D, Adnan K, et al. The Use and Efficacy of FFR-CT: Real-World Multicenter Audit of Clinical Data With Cost Analysis. JACC Cardiovasc Imaging. 2023;16:1056-65. https://doi.org/10.1016/j.jcmg.2023.02.005 [ Links ]
11. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291-300. https://doi.org/10.1056/NEJMoa1415516 [ Links ]
12. Spertus JA, Jones PG, Maron DJ, O'Brien SM, Reynolds HR, Rosenberg Y, et al. ISCHEMIA Research Group. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382:1408-19. https://doi.org/10.1056/NEJMoa1916370 [ Links ]
13. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. https://doi.org/10.1056/NEJMoa1205361 [ Links ]
14. Bittencourt MS, Hulten EA, Murthy VL, Cheezum M, Rochitte CE, Di Carli MF, et al. Clinical Outcomes After Evaluation of Stable Chest Pain by Coronary Computed Tomographic Angiography Versus Usual Care: A Meta-Analysis. Circ Cardiovasc Imaging. 2016;9:e004419. https://doi.org/10.1161/CIRCIMAGING.115.004419 [ Links ]
15. Rozanski A, Miller RJH, Gransar H, Han D, Slomka P, Dey D, et al. Benefit of Early Revascularization Based on Inducible Ischemia and Left Ventricular Ejection Fraction. J Am Coll Cardiol. 2022;80:202-15. https://doi.org/10.1016/j.jacc.2022.04.052 [ Links ]
16. Patel KK, Spertus JA, Chan PS, Sperry BW, Thompson RC, Al Badarin F, et al. Extent of Myocardial Ischemia on Positron Emission Tomography and Survival Benefit With Early Revascularization. J Am Coll Cardiol. 2019;74:1645-54. https://doi.org/10.1016/j.jacc.2019.07.055 [ Links ]
17. Azadani PN, Miller RJH, Sharir T, Diniz MA, Hu LH, Otaki Y, et al. Impact of Early Revascularization on Major Adverse Cardiovascular Events in Relation to Automatically Quantified Ischemia. JACC Cardiovasc Imaging. 2021;14:644-53. https://doi.org/10.1016/j.jcmg.2020.05.039 [ Links ]
18. Mancini GBJ, Hartigan PM, Shaw LJ, Berman DS, Hayes SW, Bates ER, et al. Predicting outcome in the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation): coronary anatomy versus ischemia. JACC Cardiovasc Interv. 2014;7:195-201. https://doi.org/10.1016/j.jcin.2013.10.017 [ Links ]
19. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2023 ;148:e9-e119. https://doi.org/10.1161/CIR.0000000000001168 [ Links ]
20. Thygesen K, Alpert J, Jaffe A, Chaitman B, Bax J, Morrow D, et al. Consenso ESC 2018 sobre la cuarta definición universal del infarto de miocardio. Rev Esp Cardiol. 2019;72:1-27 [ Links ]
21. Levy BI, Heusch G, Camici PG. The many faces of myocardial ischemia and angina. Cardiovasc Res. 2019;115:1460-70. https://doi.org/10.1093/cvr/cvz160 [ Links ]
22. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, et al. Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int J Cardiol. 2018;250:16-20. https://doi.org/10.1016/j.ijcard.2017.08.068 [ Links ]
23. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention. 2021;16:1049-69. https://doi.org/10.4244/EIJY20M07_01 [ Links ]
24. Ford TJ, Ong P, Sechtem U, Beltrame J, Camici PG, Crea F, et al. COVADIS Study Group. Assessment of Vascular Dysfunction in Patients Without Obstructive Coronary Artery Disease: Why, How, and When. JACC Cardiovasc Interv. 2020;13:1847-64. https://doi.org/10.1016/j.jcin.2020.05.052 [ Links ]
25. Gobbo M, Meretta A, Sciancalepore MA, Retamozo E, Beber E, Rosa D, y cols. INOCA: Evaluación no invasiva de los mecanismos fisiopatológicos mediante CZT-SPECT. Rev Argen Cardiol 2022; 90: 194-202. http://dx.doi.org/10.7775/rac.es.v90.i3.20515 [ Links ]
26. Camici PG, d'Amati G, Rimoldi O. Coronary microvascular dysfunction: mechanisms and functional assessment. Nat Rev Cardiol. 2015;12:48-62. https://doi.org/10.1038/nrcardio.2014.160 [ Links ]
27. Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19-27. https://doi.org/10.1161/CIRCULATIONAHA.114.011939 [ Links ]
28. Blanco J, Redruello M, Collaud Carlos; Barcellos R; Pabstleben N; Brodsky L y col. Detección de isquemia post revascularización por estudios de Perfusión Miocárdica SPECT. Resúmenes de temas Libres, SAC 2016. [ Links ]
29. Zellweger MJ, Weinbacher M, Zutter AW, Jeger RV, Mueller- Brand J, Kaiser C, et al. Long-term outcome of patients with silent versus symptomatic ischemia six months after percutaneous coronary intervention and stenting. J Am Coll Cardiol. 2003;42:33-40. https://doi.org/10.1016/s0735-1097(03)00557-6 [ Links ]
30. Patel KK, Spertus JA, Arnold SV, Chan PS, Kennedy KF, Jones PG, et al. Ischemia on PET MPI May Identify Patients With Improvement in Angina and Health Status Post-Revascularization. J Am Coll Cardiol. 2019;74:1734-6. https://doi.org/10.1016/j.jacc.2019.06.074 [ Links ]
Received: October 05, 2023; Accepted: December 07, 2023











 texto em
texto em 



