Moreover, the ACCORD trial had to be interrupted due to an increase in the total mortality in the arm of patients with type 2 diabetes treated with intensified strategy. Due to the uncertainty of the cardiovascular safety of some antihyperglycemic drugs,in 2008 the United States Food and Drug Administration issued a guideline adressing the pharmaceutical industry in wich they established that the main requirement for the approval of a new drug was that it does not demonstrate an inacceptable rise in the cardiovascular risk4
After that the European Medical Agency and other regulatories entities published similar guidelines5 that focused on the next points:
- In researchs in phase 2-3 ,the adjudication of adverse cardiovascular events must be prospective and independent in order to facilitate a metaanálysis of these events in all the controled studies of drugs versus placebo, drug plus standard therapy ( add on ) or with active comparators.
-The mayor cardiovascular end points defined as cardiovascular mortality, acute myocardial infarction and stroke(MACE),can be also evaluated with other criteria such as hospitalization due to unstable angina,revascularization procedures or heart failure (extended MACE of 4 points)
- A population of high risk of cardiovascular events ( patients with type 2 diabetes of long duration, old patients or those with kidney failure)must be included and the drug safety must be assessed for at least for two years.
Using these criteria three possibles scenarios were described:
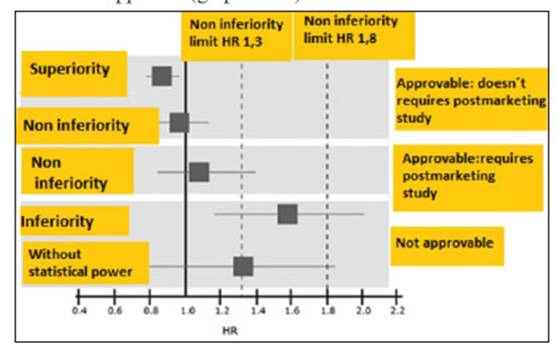
1) If the risk evaluation results in a Hazard ratio without differences between the groups but the superior arm of the confidence Interval 95% is below < 1,3 or if there is a HR of superiority ( < 1) the drug should be approved and it doesn't requires further analyisis post marketing
2) If the risk evaluation results in a Hazard ratio without differences between the groups ,but the superior arm of the confidence Interval 95% is between >1,3 and less than 1,8 the drug can be approved,but a safety cardiovascular study is required post marketing
3) If the risk evaluation concludes that there is a Hazard ratio with no statistial power but the superior arm of the confidence Interval 95% is superior to 1,8 ,or results inferior than the comparator (more risk) the drug should not be approved (graphic N 1)
In the next decade , a new generation of researches were carried out using another class of drugs., Despite not obtaining such a great decrease in the HbAlc as the previous studies , a significant reducction in the events of cardiovascular mortality and mayor cardiovascular events ( myocardial infarction and stroke) was demonstrated.
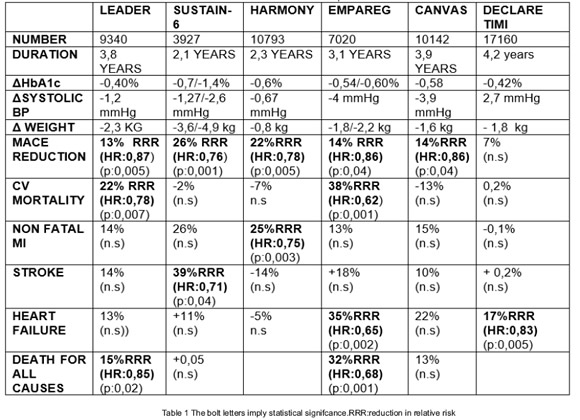
Two class of drugs were used in these studies : the SGLT2 drugs and the analogs like glucagón peptide (GLP1) drugs (Table N 1). Using the first class of drugs,:three important studies : the EMPAREG 6, the CANVAS 7 study and recently the DECLARE 8study obtained a reduction in the some of the three major adverse events (MACE) established or a significant reduction in events regarding heart failure.
When the second class of drugs were used :three major studies,the LEADER 9, the SUSTAIN 610 and the HARMONY 11 study demonstrated a significant reduction in the number of events of cardiovascular mortality ,or a decrease in the frequency of adverse events of myocardial infarction, and stroke.
So ,taking into consideration the results described above ,what are the main known effects of the glucagón like peptide agonist over the cardiovascular system?
At least , we can consider the following ones:
1) Expresion of the GLP1 receptor in the left atrium
2) Direct action over cardiomyocites
3) Antiinflamatory effects of the GLP1
4) Action of the GLP1 agonist on the endothelium cells
5) Action of the GLP1 agonist on the renin,angiotensin, aldosterone system
6) Natriuretic effect of the GLP1 agonist
7) Action on the atrial natriuretic peptide
8) Stabilization of the atherosclerotic plaque
9) Reducction in systolic and diastolic blood pressure.
10) Effects on lipids
11) Reduction of the hypoglycemic risk
ATRIUM
Both In the hearts of non human primates and in human heart , the receptors of GLP1 agonist have only been identified in the auricular area close to the sinoauricular node 12 Nevertheless , some studies have detected partial GLP1 R mRNA transcripts by reverse transcriptase polymerase chain reaction techniques in the left ventricule.13
The clinical use of GLP1 agonist has been related to the rise in the heart rate in several studies.Researchers that have using 24hs monitoring shows that the variation in the heart rate depends on the class of GLP1 agonist that is used:long acting GLP1 agonist elevate the heart rate for 24 hs ,whereas short acting drugs only lead to a transient rise in the heart rate .The relative contribution of the autonomic nervous system versus the direct effect of the GLP1 agonist over the GLP1R located in the proximity of the sinoauricular node is difficult to discern14
2) DIRECT ACTION OVER CARDIOMYOCITES
Adult rats cardiomyocyites treated with native GLP1 increases cyclic AMP levels,so is clear that their action is mediated by coupling with G Protein in a path similar to the Beta cell in pancreas 15 Exposition of neonatal cardiomyocytes to exendin -4 increases Akt and extracelular regulated kinase (ERK) phosphorylation 16,this action may reduce celular apoptosis after episodes of hipoxia/reoxigenation.
In models of infarction in mouse C57BL/ 6 in wich the main left coronary artery was ligated ,the administration of a dose of 75-200 yg of liraglutide reduced its size in comparison to controls suggesting a direct effect over the affected myocardiocites . Liraglutide also modulated the expression and the activity of cardioprotective genes and proteins in the mouse heart,including Akt,GSK3,PPARp5,NrF2 and HO 17
Adittional studies with albiglutide, lixisenatide and exenatide demonstrated similar effects , and the cardioprotective action can be inhibited with the use of a specific inhibitor such as GLP1-RA exendin(9-39) .This suggested a direct action over the canonical GLP1 receptor 18
At pharmacological dose ,intravenous GLP1 dose improved left ventricular function ,máximum oxigen uptake and physical performance in subjetcs with heart failure. Similarly ,intravenous exenatide reduce wedge pressure, and improved parameters of cardiac index , with better contractility however these facts were founded in vitro only in atrial cardiomyocites but not in ventricular myocardium19
In the case of human diabetic subjects with acute myocardial infarction with ST-T elevation who undertook primary percutaneous coronary intervention ,the treatment with liraglutide improved left ventricular ejection fraction at 3 months compared to placebo,reducing the hs CPR ,and increased the activity of endotelial Oxide nítric syntetase20 Nevertheless ,while in the Leader study,there was a non significant reduction of 13% in the frequency of heart failure, in a study that lasted 12 weeks with less number of patients,Lepore et al did not find an improvement in left ventricular ejection fraction , 6 minutes work testing ,left ventricular structure and function,and parameters of glucose and oxygen utilization in obese and overweight patients with class II-III NYHA heart failure and ejection fraction less tan 40% with the use of albiglutide versus placebo 21 With similar findings, the FIGHT study 22 carried out in patients with severe heart failure ( FE <26%) or history of previous hospitalization for heart failure during the former 180 days,did not find any significant improvement with the use of liraglutide compared to placebo regarding hospitalizations for new events of heart failure, mortality and levels of NTproBNP.More patients in the group liraglutide were rehospitalized due to heart failure.
Likewise,In the LIVE STUDY , in diabetic and non diabetic patients with stable heart failure (EF less of 45%)no improvements were founded with the use of liraglutide vs placebo over the ventricular systolic function and serious events cardiac evens were superior in the first arm . 23
Therefore ,while there is not a clear evidence in favor of the use of GLP1 agonist in the setting of severe heart failure, a study carried out with a large number of high risk patients as the LEADER study have not demonstrated an increase in the risk of it . Conversely an inverse tendency was found in most of them.
3) ANTIINFLAMATORY ACTION OF GLP1 ANALOGS
The enteroendocrine L cells can secrete GLP1 in response to proinflamatory stimuli .
This can in turn act promoting the expresión of GLP1 receptors over the intraepthelial limphocytes (iLES) with less locally intestinal inflamation.
GLP-1 may also indirectly reduce inflammation in different peripheral organs through weight loss or improved glucose control, or by targeting GLP-1Rs expressed on populations of circulating immune cells. Alternatively, GLP-1R activation may directly reduce inflammation in organs and cell types expressing the GLP-1R.
The gut has been found to be the biggest center of interrelation between the intestinal microflora and the immnune system and an important target for the control of inflammation impacting not only in the local gut mucose ,but also in distant organs including the liver ,adiposse tissue,muscle, blood vessels, heart and pancreatic islets.Similarly,the enteroendocrine cells reprent the largest specialized endocrine networks in human physiology24
In the case of rodents exposed to intestinal inflammation through the administration of lypopolysaccarides ,the levels of derivates of proglucagon derived peptides are increased .Interleukin 6 and LPS directly influence the increase in the secretion ofGLP1 from a line ofmurine gut L cells25
The importance of basal intestinal GLP1 signaling for control of local inflammatory signals was demonstrated in GLP1 -/- knock out mice models in wich exhibited gut microbial dysbiosis and markedly increased sensitivity to experimental induced colonic inflammation was observed 26
The levels of GLP1 peptides are 6,9 fold elevated in critical care patients , and in those patients it can help predict the risk of mortality in sepsis and critical illnes27
Anti-inflammatory effects of GLP-1R agonists were also demonstrated using human aortic endothelial cells(HAECs) pre-treated with liraglutide for 1 hr,and then exposed to TNF-a or LPS plus liraglutide for 3 hr. The induction of vascular cell adhesion molecule-1 (VCAM-1) and E-selectin expression was attenuated, and adhesion of THP-1 monocytes to HAECs was reduced using liraglutide.These actions required increases in intracellular Ca2+, Ca2+/calmodulin-dependent protein kinase (CAMK)-, and AMPK-dependent signaling28
The GLP1 receptor agonist liraglutide and semaglutide significantly attenuated plaque lesion development in apolipoprotein E deficient mice (Apo E-/-)and low density receptor lipoprotein receptor (LDLr-/-)mice, and this attenuation was independent of colesterol and weight lowering.This fact suggested a direct antiinflamatory action in the plaque tissue related to less leukocyte recruitment ,reducción in leuckocyte rolling,adhesión and extravasation , changes in the turnover of extracelular matrix proteins( metaloproteins),or reduction in the risk of plaque hemorrhage 29
Nevertheless the expression of GLP1 R canonical receptor in mouse peritoneal or tissue macrophages are not fully described. So, the precise mechanism through the GLP1 modified immune systemic inflammation needs to be dilucidated30
4) ACTION OF GLP1 OVER ENDOTHELIUM CELLS
Some pioneer studies have been suggesting the necessary presence of GLP1 receptors in human coronary endohelial cells .It was observed that the incubation of those cells with exendin-4 stimulates proliferation of human coronary artery endothelial cells and at the same time the activation of oxide nitric synthetase (eNOs) ,MAP kinasa and PI3K/AKT dependent pathway . 31 Also, in human umbilical endothelial cells
(HUVEC) some results were published wich concluded that exenatide exerts direct protective effects on endothelial cells through the AMP/Akt/eNOs pathway in a GPL1 receptor dose dependent manner.32
However,the real presence of the GLP1 receptor in the coronary endhotelial cells was questioned .The GLP1 receptor was cloned in 1992 and most of the researchers used inmunochemistry methods.But when a careful Western Blotting characterization of multiple available polyclonal antibodies (Pba) against the GLP1 was done, the authors concluded that several of them did not detect authentic GLP1-R protein , even they used optimal enhanced methods.33 After that a rigorous validation criteria for the use of any antiGPLR antibody was defined 34
The native GLP1,once modified by the iddp4 enzyme, present in the coronary endothelium , could have some vasodilatadory action mediated by their metabolites GLP1 (9-36) and (7-36) wich may increased blood flow with independence from the presence of GLP1 receptor.35
Despite these observations , in a randomized study on obese patients with prediabetes, the endothelial function assessed by digital reactive hyperemia ,did not show any significant differency with the use of exenatide in comparison with metformin. 36 Likewise, there were not differences over the endothelial function measured by flow mediated dilation(FMD) in diabetic patients in a study comparing the action of liraglutide vs glargine insulin 37
5) ACTION OF GLP1 OVER THE RENIN ANGIOTENSINALDOSTERONE SYSTEM
Using a new validated monoclonal antibody Pykes and cols. found the presence of GLP1R activity in smooth vascular muscle of the afferent arteriole closely to cells of the yuxtamedular complex 38 with coexpressed simultaneous inmmunoreactivity for renin in normal primate and human kidney.
In a randomized,controlled ,double-blinded ,single day study over twelve healthy patients,the 2 hs infusión of GLP1 had little effect over the glomerular filtration rate and the renal plasma flow compared to placebo. Nevertheless the renal clearance of sodium rose 40% and the concentration of angiotensin II decreased significantly (19%) whereas renin,aldosterone and the urinary excretion of angiotensinogen showed no significant changes. 39
6) NATRIURETIC EFFECT OF GLP1
The GLP1 induced natriuretic effects in healthy subjects and in insulin resistant obese men.Intravenous infusion of GLP1 enhance sodium excretion,reduce H+ secretion and reduce glomerular hyperflitration in obese men .These finding suggest an action at the proximal renal tubule and a potential renoprotective effect. 40
7) ACTION ON THE ATRIAL NATRIURETIC FACTOR
In mice,after the infusion of angiotensin II to raise their blood pressure, the administration of GLP1 like peptide analogs augmented the secretion of atrial natriuretic peptide (ANP) through the activation of GLP1 receptor.41 determining a decrease in it .But this effect is only observed in wild and not in KO GLP1 receptor mice.
Therefore, this raised the possibility of an axis entero-atrial that explain the mayor cardiovascular effects of GLP1 analogs:the GLP1 secreted by L cells in the gut binds to GLP1 R in the atrial myocardium and induced ANP secretion via Epac 2 .At the same time, ANP reduced blood pressure by increasing the permeability of blood vessels,inducing vasorelaxation and enhancing sodium secretion and inhibiting sodium reabsortion in the kidney. An additional mechanism of ANP mediated natriuresis includes increases in glomerular filtration rate, direct inhibition of renin,and supression of aldosterone secretion from the adrenal zona glomerulose cells.42
Nevertheless ,in a study with a relative small number of hypertensive subjects with diabetes, liraglutide promoted natriuresis but did not increase circulating levels of ANP. 43 after 3 weeks of treatment. In another research a specific relationship between natriuresis and levels of pro atrial natriuretic peptide in healthy males was not found either.44
As a consequence, although it is clear that analogs glucagón like peptides promotes natriuresis their specific relationship with hemodynamic action peptides requires further researchs and explanations.
Even so , could there be another action attributable to the atrial natriuretic factor?
Some authors have demonstrated that the changes in the body composition and the magnitude of weight loss in obese diabetic patients induced by liraglutide are related to the levels of the ANPI 45 Likewise,in an elegant ANP receptor knock out mouse model and a line of human adypocites cells , Bordiccia and cols have demonstrated that in mouse and human adypocites Atrial natriuretic peptide (ANP) and ventricular natriuretic peptide(BNP) activated PPAR X coactivator 1 a (PGC-1a) and mitochondrial uncoupling protein 1 (UCP1) expresión, induced mitochondriogenesis and increased uncoupled and total respiration.This effect results in increased lipolysis and energy expenditure,promoting “browning” of the white adipose tissue.This mechanism defines the heart as a central regulator of the adipose tissue biology .46 In this sense, a potencial action of the peptides ANP and BNP is that they could act as thermodynamic regulators dissipating much of the energy as heat and relieving cardiac work as a consequence.
8) STABILIZATION OF THE ATHEROSCLEROTICPLAQUE PROGRESSIONIn a apolipoprotein E deficient (apoE-/-) mouse model the treatment with liraglutide inhibited the progression of early onset atherosclerotic plaques and enhance plaque stability core 47 It has also been confirmed that Lixisenatide confer protection against atherosclerosis: 2 months subcutaneous infusion of this compound decreased aortic arch lesión size in high cholesterol fed Apo E-/- mice that were also heterozygous for insulin substrate 2 protein 48 Lixisenatide also improved plaque stability as determined by plaque collagen content and increased plaque fibrous cap thickness. Treatment of mouse aortic VSMC with exendin-4 reduced platelet derived growth factor that induced cell proliferation factor49 In addition,liraglutide reduced oxidized low density lipoprotein-induced mitochondrial reactive oxygen species(ROS) generation in aortic human vascular smooth muscle cells. 50 In a study of patient who were submitted to carotid endartherectomy, atherosclerotic plaques were obtained from diabetics in comparison with no diabetics patients.Likewise,the plaques were compared between those diabetics patients treated with analogs glucagón like peptide or Iddp4 drugs versus non treated patients.Compared with plaques in non diabétic patients, diabetic plaques had more inflammation and oxidative stress along with less SIRT6 ( sirtuins) expresión and collagen content.In those diabetics patient treated with GLP1 therapy, the plaques presented greater SIRT6 expression and less inflammation and oxidative stress ,indicating more stable plaque phenotype in comparison with non treated diabetic patients.51
9) REDUCTION IN SYSTOLIC AND DIASTOLIC BLOODPRESSUREIn a metaanálisis Visboll et al. 52 have founded a reduction in the systolic and diastolic blood pressure in the diabetic patients treated with GLP1 analogs in comparison with other therapies.In other metaanálisis Wang and cols 53 founded that the treatment with liraglutide and exenatide reduced 1 to 5 mmHg the systolic and diastolic blood pressure compared with some others anti diabetic drugs including insulin, glimepiride and placebo.In a pooled analysys of six randomized clinical trials done over 2792 patients,the mean reduction of the systolic blood pressure and diastolic blood pressure with the use of liraglutide in comparison to placebo were evident after 2 weeks and mantain the effect a 26 weeks,the reduction was
10) EFFECTS ON LIPIDS
GLP1 R agonist reduce intestinal chylomicron production and secretion in normoglycemic and diabetic rodents and humans , and it has been shown that it also reduce postprandial triglycerides and apo B48.Some studies carried out over animals and humans have demonstrated reduction of liver fat and hepatic lipoprotein synthesis and secretion with these compounds.Nevertheless, neiter enterocytes, the mayor cell type responsible for chylomicron synthesis and secretion and nor the hepatocytes express canonical GLP1 R 62 so it is possible that their action is produced by an indirect mecanism.
In type 2 diabetics ,liraglutide lowered the average postprandial triglycerides profile in comparison with placebo. 63 In the LEAD studies 1 to 6 the levels of total cholesterol, LDL cholesterol and tryglicerides were significantly reduced in type 2 diabetics patients treated with liraglutide in dose of 1.8 mg compared with rosiglitazone, glimepiride, glargine insulina or exenatide.64
In an observational real life study that lasted 18th months in wich one hundred twenty one subjects with diabetes and metabolic syndrome were studied , the addition of liraglutide to metformin was associated with a reduccion in the levels of total and LDL cholesterol and tryglicerides and in the carotid intima thickness assessed by B mode real time ultrasound .At the end of the observation period (18 months) 26% of the patients no longer fulfilled the criteria of metabolic syndrome65
In the SCALE ,a fifty six week, double blinded,placebo controled study,the levels of total cholesterol,VLDL,HDL and tryglicerides were significantly reduced with the dose of 3 mg liraglutide but not with 1,8 mg.No effects on LDL cholesterol or free fatty acids were observed. 66
Nevertheless, in the largest 3,8 years LEADER study not significant differences were observed between the liraglutide and placebo arm in terms of total colesterol,HDL cholesterol, tryglicerides and LDL cholesterol. 9 Conversely in the EXSCEL study, in wich exenatide was used once week a slight but significant difference was observed in the levels of low density cholesterol,(1,5 mg/dl or 0,04 mmol/l) and tryglicerides (1,8 mg or -0,02 mmol/l) between the treatment versus placebo arm. 67
In the SUSTAIN 6 (10 study the difference of total cholesterol between the treatment with semagludie and placebo arm were
11) REDUCTION OF THE HYPOGLYCEMIC RISKIn the LEADER
study , the frequency of hypoglycemic events was 20% inferior in the group assigned to liraglutide versus the control group,and the frequency of severe hypoglycemia was 31% less,wich was highly significant. 9 In the
Exscel study using exenatide once a week there no were significant differences in the rate of severe hypoglucemic episodes between the treated and placebo arm. 67 In the ELIXA study with lixisenatide vs placebo in type 2 diabetics patients with and acute coronary syndrome, 16,6% of hypoglycemic episodes were reported in the lixisenatide group and 15,2% in the placebo group without statistical significancy, but serious hypoglucemic episodes ( that required assistance of a third person) were numerically less frequent with lixisenatide (14 patients reporting 16 events) than with placebo (24 patients reported 37 events) (68)
In the Harmony trial with a randomization of type 2 diabetes patients to albiglutide once a week versus placebo, the events of severe hypoglucemia were 44% inferior en the albiglutide arm (31 patients vs 55 patients) 11)
In a post hoc análysis made by Zinmann et al. over the LEADER study they found that 114 patients experienced severe hypoglycemia in the liraglutide arm vs 154 in the placebo arm ( 31% less) .In both arms, the patients who suffered severe hypoglycemia had longer diabetes duration, higher incidence of heart failure and use insulin more frequently at baseline.The most important fact about patients regardless they were treated or not was that those who were experiencing severe hypoglycemia were at greater risk of cardiovascular events and death, particularty shortly after of the hypoglycemic episode. A clear temporal association was observed between severe hypoglycemic episodes and subsequent risk of mayor adverse cardiovascular events (MACE),cardiovascular death ,non cardiovascular death or all cause death 69 These findings were similar to those described by Zoungas et al in the ADVANCE study 70 In a recent metaanálisis and systematic revisión,Fizpatrick et al. found a definitive association between hypoglucemia and cardiac arrhythmia. The hypoglycemia was associated to a less variation of the cardiac rhytm and mayor frequency of arrhytmia.Moreover ,the QTc interval was longer during the hypoglycaemic events than during the euglucemic intervals. 71
CAN WE SPEAK OF A CLASS EFFECT?
The GLP1 analogs differ in some pharmacokinetics and pharmacodynamics characteristics.
Exenatide and lixisenatide share a 53% and 50% aminoacid homology respectively with the native GLP1 72,while liraglutide and semaglutide share an 97% and 94% of aminoacid homology respectively with the GLP1 native 73 At the same time, albiglutide and dulaglutide share an 94% and 91% of aminoacid homology respectively with the native GLP1. Extensive research has been carried out in the last years in order to look for some molecular characteristics that determine that the compounds have a greater power as GLP1 receptor agonist such the synthesis of small molecules GLP1 agonist and some alosteric modulators, biased agonist and triagonist. 74
Also, some pharmacodynamic characteristics may influence the differential action of GLP1 agonist :whereas exenatide and lixisenatide has a brief duration of action(2-5 hours) , liraglutide has an average half life of 13 hours and is suitable for once daily administration 75 ,and exenatide extended release and albiglutide can last up to a week 76
Liraglutide has the addition of a C16 fatty acid that allows reversible binding to albumin wich slows degradation and reduces renal clearance.Additionally, it results in increased self association ( wich slows absortion from the subcutaneous depot ) ,reduced susceptibility to DPP-4( dipeptidyl peptidase -4) and prolongs its plasma half life ,protacting its action.Semaglutide has the addition of a C-18 di acid wich allows reversible binding to albumin,wich slows degradation and reduces renal clearance.The aminoacid sustitution at position 8 protects against DPP4 degradation.Albiglutide has two GLP1 molecules attached to albumin to retain its therapeutic effect and a aminoacid substitution at position 8 that protects against DPP4 degradation while dulaglutide has two GLP1 DPP4 resistant molécules covalently linked to a modified IgG4 Fragment thas acts as carrier limiting the renal clearance of the molecule. 77
If those characteristics has some effect on the cardiovascular outcomes is still a source of further debate.
CARDIOVASCULAR OUTCOMESTo date there are five major investigations that have explored the efficacy and safety of glucagón like peptide analogues in patients with high cardiovascular risk.
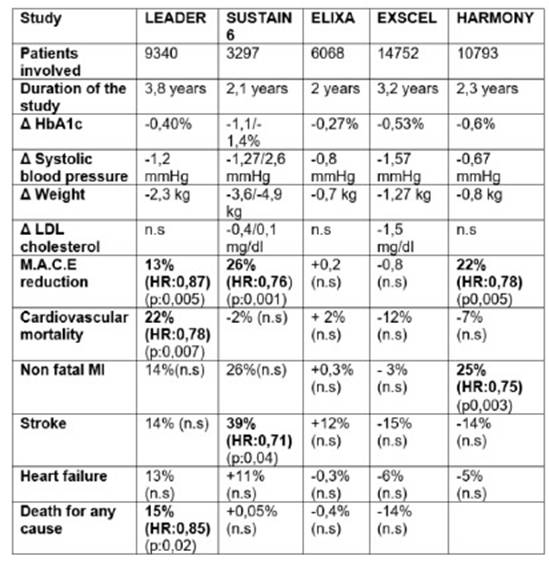
In the ELIXA trial 6068 patients were included ,with antecedents of myocardial infarction or hospitalization for inestable angina in the last 180 days.They were randomized to receive lixisenatide or placebo and the study lasted two years.During the observation period the HbA1c was 0,27% inferior on average in the lixisenatide arm vs the placebo arm, the differences in systolic blood pressure between the two groups was 0,8 mmHg and the group assigned to lixisenatide had a loss of 0,7 kg weight regarding the placebo arm.The end compositum objetive was to evaluate the incidence of cardiovascular death,myocardial infarction,stroke or hospitalization for inestable angine ( MACE) .13%(406 patients) of the patients in the lixisenatide arm versus 13,2% of those in the placebo groups ( 309 patients ) suffered some of this events wich confim the non inferiority but not the superiority of the compound in relation to placebo.Also there were not diferences in relation to heart failure or death for all causes. (68 The LEADER trial was a randomized, double blind study, the most extensive in terms of patients follow up:it lasted 3,8 years and it was also the one with the largest number of patients involved ( 9340 patients).
81,3% of the patients had high cardiovascular risk (previous non fatal myocardial infarction,stroke and/or renal failure with creatinine clearence less than 60 ml/min or heart failure in NYHA stage II-III). 18,7% of the patients were with high cardiovascular risk (micro and/or macroproteinuria,arterial hypertension or left ventricular hypertrophy,ventricular diastolic and/or systolic dysfunction and and ankle/arm index less than 0,9)
The average difference in HbA1c between the group asigned to liraglutide versus placebo was 0,40% .The systolic blood pressure was 1,2 mmHg lowest in the liraglutide arm and, in average, the patients in this arm lost 2,3 kg more than patients in placebo .Also,there was a 13% reducction in the mayor cardiovascular combinated events (MACE),wich was highly statistical significant.608 events in 4668 patients in the liraglutide arm vs 694 events in 4672 patients(14,9%) in the placebo arm .(Hazard ratio 0,87;95% confidence Interval (CI) 0,78 to 0,97, p<0,001 for non inferiority ,p:0,01 for superiority).The cardiovascular mortality was 22% inferior in the liraglutide arm than in the placebo,also highly significant.(Hazard ratio:0,78;95% CI 0,66 to 0,93 p:0,007). There was a non significant reduction in the number of non fatal myocardial infarctions ( 14%), stroke (14%) and hospitalization for heart failure in the liraglutide arm.
Regarding death by any causes, the patients assigned to the liraglutide group showed a significant reduction of 15% during the observational period (381 patients 8.2%) compared to the placebo group (447 9.6%) (hazard ratio, 0.85; 95% CI, 0.74 to 0.97; P=0.02)
In the subgroup análysis, those patients with a glomerular filtration rate less than 60 ml/min benefitted more from the active treatment.(33% less MACE events vs placebo ) than those with normal glomerular filtration rate. (hazard ratio:0,67 ;95% IC:0,54-0,83).The most common cause leading to discontinuation of the drug were gastrointestinal effects, and the incidence of pancreatitis was non significantly lower in the liraglutide group than in the placebo group .There were more episodes of gallstone disease with liraglutide, a finding that was previously reported.Also,there was a higher rate of retinopathy events with liraglutide than with placebo, although the difference was not significant.
The study demonstrate superiority of liraglutide compared to placebo in terms of reduction of mayor cardiovascular events,cardiovascular mortality and death of all causes The number of patients who would need to be treated to prevent one event in 3 years was 66 in the analysis of the primary outcome and 98 in the analysis of death from any cause (9
In the EXSCEL TRIAL 14752 type 2 diabetic patiens of whom the 73% had previous cardiovascular disease were followed for a median of 3,2 years.This was a randomized, doble blind trial with the use of extended release exenatide at dose of 2 mg weekly versus a placebo controled arm.Again, the primary outcome was the first occurrence of death from cardiovascular causes,non fatal myocardial infarction or non fatal stroke (MACE outcome) Secondary outcomes included death for any causes, death from cardiovascular causes,and the first occurrence of non fatal or fatal myocardial infarction, non fatal or fatal stroke, hospitalization for acute coronary syndrome and hospitalization for heart failure.
-At baseline,the median lenght of time that patients had had diabetes was 12 years , and the basal HbA1c was 8% .16,2% of the patients had a history of heart failure.During the study the difference of HbA1c between the exenatide arm vs the placebo arm was 0,53%. The exenatide group had in average less -1,5 mmHg systolic blood pressure and the patients assigned to this arm lost .1,27 kg weight regarding to placebo.11,4% of the patients in the exenatide arm ( 3,7 events persons /year) and 12,7% in the placebo arm ( 4 events persons/year) (Hazard ratio : 0,91,95% CI 0,83 to 1) suffered a primary composite event indicating that exenatide,administered once a week was no inferior to placebo with respect to safety ( p < 0,001 for non inferiority )and was not superior to placebo with respect to efficacy ( p:0,06 for superiority)
The risk of death from any cause was 6.9% in the exenatide group and 7,9% in the placebo group but this difference was not considered to be significantly on the basis of the hierarchical testing plan.
Patients randomly assigned to exenatide, administered once week had a lower risk of receiving an additional cointervention glucose lowering drug than patients in the placebo group and a lower risk of initiating long term insulin therapy.The rate of severe hypoglycemia didn'differ significantly between the two groups. The incidence of acute pancreatitis,pancreatic cancer,medulary thyroid carcinoma and serious adverse events did not differ significantly between the two groups.One of the limitations of this trial was the lost of follow up, the type 2 diabetic patients involved in the study had a mean exposure to the trial régimen of only 2,4 years with 25% of the study subjects being not fully mantained on the asigned trial régimen and more than 40% of the participants had prematurely discontinuated their assigned study medication(67)
The SUSTAIN 6 study utilized semaglutide,a prolonged action GLP1 agonist ,that allows its administration once a week in dose of 0,5 and 1 mg in comparison with placebo, in a double blind fashion.The research lasted 104 weeks (2,1 years) and included 3297 patients with high cardiovascular risk (83% with previous cardiovascular events,chronic kidney disease with the exception of patients with glomerular filtration estimated in less of 30 ml/min.) The rest of the patients had risk factors such as microalbuminuria or proteinuria,arterial hypertension or left ventricular hypertrophy, left diastolic or systolic dysfunction or ankle/ arm index less than 0,9.The average age or the patients was 65 years old,the overall duration of type 2 diabetes was 13,9 years and the mean glycated hemoglobin levels was 8,7% at the beginning.
The average difference in HbA1c between the 0,5 mg and 1 mg semaglutide arm in relation to placebo was -1,1% and 1,4% respectively.The systolic blood pressure was -1,27 mmHg and -2,6 mmHg inferior in the 0,5 and 1 mg arm.The 0,5 mg semaglutide arm loss 3,6 kg and the arm 1 mg -4,9 kg weight in comparison to placebo.
The primary composite outcome was the first ocurrence of cardiovascular death, non fatal myocardial infarcion or non fatal stroke .(MACE) The primary outcome ocurred 26% less in the semaglutide treated patients : 108 of 1648 patients (6,6%) in the semaglutide group and 146 of 1649 patients (8,9%) in the placebo group,suffered one event. ( Hazard ratio 0,74%,95% CI 0,85-0,95 p< 0,001 for non inferiority and p<0,02 for superiority).It was necesary to treat 41 patients to reduce one primary composite outcome.The reduction of the primary objective was leaded by a significant reduction in the frequency of non fatal stroke in the patients assigned to the semaglutide arm:non fatal stroke occurred in 27 patients ( 1,6%) in the semaglutide group and 44( 2,7%) in the placebo group ( Hazard ratio 0,61;95% CI 0,38 to 0,99 p:0,04)
Non fatal myocardial infarction ocurred in 47 patients (2,9%) in the semaglutide group and 64 (3,9%)in the placebo group , a difference that was not statistically significant.The risk of cardiovascular death was similar in both groups ,44 patients in the semaglutide group (2,7%) and 46 patients( 2,8%) in the placebo group, also non significant.
The new or worsening nephrophaty was reduced in 36% in the patients treated with semaglutide but there were an 76% increment in the diabetic retinopathy: 50 patients in the semaglutide group (3%) vs 29 patiens in the placebo group (1,8%) suffered some diabetic retinopathy complications ( need of retinal photocoagulation, use of intravitreal agents,vitreous hemorrhage or onset of diabetes related blindness) 69 There were a non significant raise in heart failure in both semaglutide arms (14%) and there was not differences in the mortality by all causes between the semaglutide and placebo treated.There were more pancreatitis episodes and pancreatic cancer in the placebo arm than in both semaglutide dose arms .The gallblader disorder was similar in all groups.(10)
The last study published was the HARMONY TRIAL in wich were involved 610 sites in 28 countries.Type 2 diabetes patients aged 40 years old and older with cardiovascular disease( myocardial infarction or at least 50% stenosis in one coronary artery or more ,or previous coronary revascularization or cerebrovascular stroke or carotid stenosis or with intermitent claudication or ankle /arm index less than 0,9 or those whose received an peripheral vascular procedure) were submited to a treatment with subcutaneous once week albiglutide or a placebo in addition to their standard care.The primary outcome were similar to the previous trial ( MACE reduction).The mean age of the patients was 64,1 year,the average duration of diabetes was 14,1 years and the mean HbA1c was 8,7% .71% of the patients had a history of coronary artery disease ,25% had peripheral artery disease, 25%had previos cerebrovascular disease and 20% had a history of heart failure.The median duration of follow up was 1,6 years (máximum 2,6 years) Study treatment drug was taken for 87%of the total follow up for cardiovascular outcome in the albiglutide group and 85%of that time in the placebo group.
The mean difference of the HbA1c was 0,6% less in the albiglutide group in relation to placebo,the systolic blood pressure was 0,6 mmHg inferior in the first arm and the patients asigned to albiglutide loss in average 0,8 kg of body weight.The primary composite end point occurred in 338 (7%)of the 4731 patiens assigned to albiglutide (4,57 events per 100 patients year) and in 428 (9%) of 4372 patients in the placebo group (5,87 events per 100 patients/year) wich means a 22% reduction in the risk (HR 0,78 95% CI0,68-0,90).Albiglutide was not inferior to placebo for cardiovascular safety ( p<0,0001 for non inferiority)and superior to placebo for efficacy (p:0,0006).This means that we need to treat 50 patients during 1,6 years for reducing one event.There was a significant reduction in the incidence of myocardial infarction of 25%(HR:0,61-0,90.95%CI p:0,003).Also there was a non significant reduction in the incidence of stroke events ( HR:0,86 CI95%,66 to 1,14) and death for cardiovascular causes.Neither was a difference in relation to a hospitalization for heart failure The frequency of severe hypoglycemia was low in the albiglutide and placebo arm and the number of pancreatitis and pancreatic cancer was less than 1% in both arms.There not where registry of medulary thyroid cancer.
There was not reporting of new or worsening retinopathy in the albiglutide group(11) .
In the table 2 it is described a resume of this trials.
In a recent metaanalysis and systematic revisión of twelve research identified in wich only fourth of the trial described here were selected that involved 33.457 participants ( ELIXA with lixisenatide, SUSTAIN 6 with semaglutide,LEADER with liraglutide and EXSCEL with exenatide extended released, HARMONY with albiglutide was not included) Bethel and cols. has concluded that compared with placebo,GLP1 receptor agonist showed a significant 10% relative risk reduction in the three point mayor adverse primary outcomes (MACE:cardiovascular mortality, non fatal myocardial infarction,and non fatal stroke )( HR 90 CI95% 082-0,90:0,03), a 13% reduction in relative risk of cardiovascular mortality ( HR:0,87,CI 95%0,79-0,96 p:0,07) and a 12% relative reduction in all causes mortality (HR:0,88-CI 95%:0,81-0,95 p:0,002) with low to moderate between trial statistical heterogeneity(78) Until date, no head to head cardiovascular outcomes trials have been done for this drug class,limiting detection of outcome diferences between GLP1 receptor agonist of different structures and potency.Thus their differences could be assigned to a many factors related to the drugs (solubility,release,grade of drug absortion, peak drug levels,pharmacokinetic p r o fi l e , d e g r a d a t i o n , m e t a b o l i s m , r e n a l clearance,inmunogenicity or potency on receptor signaling) either to the design of the differents trials ( population assigned,disease duration of type 2 diabetes,background therapy,length of the follow up study,size of the population studied,event adjutication,time of exposition to drugs,number of patients needed to treat to obtain a benefit,number of patients needed to treat to obtain a harm and the statystical análisis).