Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciencia y Tecnología
versão On-line ISSN 1851-7587
Rev. cienc. tecnol. no.29 Posadas jun. 2018
Corrosion Rates Evaluation of Stainless Steels in Soy Biodiesel Using Different Techniques
Evaluación de la velocidad de corrosión de aceros inoxidables en biodiesel de soja utilizando diferentes técnicas
Alejandra S. Román1,3,*, Claudia M. Méndez3, Alicia E. Ares1,2,3
1 Instituto de Materiales de Misiones, IMAM (CONICET-UNaM), Félix de Azara 1552, (3300) Posadas, Misiones, Argentina.
2 Consejo Nacional de Investigaciones Científicas y Técnicas, Godoy Cruz 2290 (C1425FQB), Buenos Aires, Argentina.
3 Facultad de Ciencias Exactas, Químicas y Naturales, Universidad Nacional de Misiones, Félix de Azara 1552, (3300) Posadas, Misiones, Argentina.
* E-mail: alejandraroman@fceqyn.unam.edu.ar
Abstract
In the present research we studied the behavior against corrosion of four stainless steels in soy biodiesel at room temperature. The stainless steels used were AISI 304L, AISI 316, Sea Cure-1 and 2205. The corrosion velocities were measured by applying electrochemical tests and weight loss methods. The electrochemical studies conducted were potentiodynamic polarization curves, measurement of the open circuit potential as a function of time, and Electrochemical Impedance Spectroscopy (EIS) measurements. In addition, gravimetric tests were performed according to ASTM G 31-72.The four stainless steels showed low corrosion rates being steel 2205 the one which always showed the lowest value. This indicates that the materials studied are suitable for use in contact with biodiesel obtained from soy oil. The agreement obtained in the results by applying different methods allows us to conclude that the electrochemical techniques are suitable to evaluate corrosion rates in soy biodiesel.
Keywords: Biodiesel; Corrosion; Stainless steels; Linear polarization; Gravimetric tests; Electrochemical Impedance Spectroscopy (EIS).
Resumen
En el presente trabajo se estudió el comportamiento frente a la corrosión de cuatro aceros inoxidables, en biodiesel de soja a temperatura ambiente. Los aceros inoxidables utilizados fueron: AISI 304L, AISI 316, Sea Cure-1 y 2205. Se midieron las velocidades de corrosión aplicando métodos electroquímicos y mediante ensayos de pérdida de peso. Los estudios electroquímicos realizados fueron curvas de polarización potenciodinámica, medidas del potencial de circuito abierto en función del tiempo, y medidas de Espectroscopía de Impedancia Electroquímica. Además, se realizaron ensayos gravimétricos siguiendo la Norma ASTM G 31-72.
Se obtuvieron bajas velocidades de corrosión para los cuatro aceros inoxidables, siendo siempre menor la velocidad de corrosión del acero 2205. Esto indica que los materiales estudiados son adecuados para utilizarse en contacto con biodiesel obtenido a partir de aceite de soja. La concordancia obtenida en los resultados al aplicar los diferentes métodos, permite concluir que los técnicas electroquímicas son adecuados para evaluar velocidades de corrosión de en biodiesel.
Palabras clave: Biodiesel; Corrosión; Aceros Inoxidables; Polarización Lineal; Ensayos Gravimétricos; Espectroscopía de Impedancia Electroquímica (EIE).
Introduction
The gradual replacement of fossil fuels by other forms of energy is a topical subject. This is due to the depletion of petrochemical reserves as well as to the awareness to preserve the environment. In this regard, biodiesel is of great interest as an ecologically sound source of energy.
The similarities of biodiesel and petroleum diesel in terms of certain properties (viscosity, density, cetane number, reaction efficiency and phase changes) allow considering this biofuel as a direct substitute for fossil fuels [1–4]. However, biodiesel also has some unfavorable characteristics such as oxidative instability, poor solvent properties at low temperatures, and lower power and torque proportion than petroleum diesel [5, 6].
Mofijur et al. [7] highlighted the importance of the compatibility of the materials and durability of engines using biodiesel, but they said that researchers are not focusing on evaluating these factors because of the higher costs and difficulties involved compared to the tests necessary to evaluate engine performance and emissions generated.
It has also been widely reported that biodiesel is more corrosive than petroleum diesel [8–13]. However, some authors believe that there is no evidence of whether or not these corrosion values are within the limits for the materials used in the automotive industry [8,14]. According to the review by Sorate & Bhale [8], studies of the corrosion behavior of materials operating in contact with biodiesel are still largely deficient.
Taking into account the possible metallic materials with which biodiesel may be in contact both in its production and storage process and during its circulation in a fuel system of a typical diesel engine, it was decided to study different stainless steels. It is known that stainless steels have good corrosion resistance in many media and their electrochemical behavior has been extensively studied [15].
Some researchers studied the compatibility of ferrous materials in biodiesel from different sources, finding different results. Studies by Maru et al. and Fernandes et al. of carbon steel in soy biodiesel and sunflower biodiesel [9, 16] demonstrated the compatibility thereof. Cursaru et al. [17] found that the degradation of carbon tempered steel in sunflower biodiesel increases with increasing temperature. Kovacs et al. [18] showed that biodiesel quality standard caused no significant effects of corrosion in storage tanks of carbon steel under normal operating conditions.
Other works evaluating different stainless steels in biodiesel produced from soy and palm oil [11, 19–22] have indicated that they are not susceptible to corrosion in the media. However, according to J. Perez- Quiróz [4], stainless steel 439 has a tendency to corrode in biodiesel. Hu et al. [23] compared the corrosive effects of rapeseed biodiesel on mild steel and stainless steel, and found that corrosion was more severe on mild steel. Jin et al. [12] found that palm biodiesel caused pitting on the mild steel ASTM 1045 and that the corrosive effect increased with temperature and exposure time.
The objective of the present work is to evaluate corrosion rates in soybean biodiesel of metallic materials using comparatively gravimetric and electrochemical techniques, seeking to offer a test methodology that allows to evaluate the compatibility of the materials with biodiesel in a fast but still effective way. The corrosion rate of a metal in a corrosive environment is usually evaluated by conducting weight loss methods [24]. These methods demand long exposure times in the corrosive system and do not allow obtaining an instantaneous corrosion rate. In contrast, electrochemical methods are fast and accurate and thus are of great importance for practical applications. These techniques require short measurement times, have virtually unlimited sensitivity, and allow continuous monitoring of corrosion. The disadvantage is that they need to disturb the system by imposing an external polarization, which can cause changes in the specific properties of the system [25].
Experimental
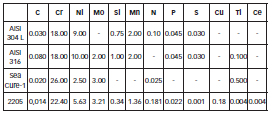
The stainless steels used were the austenitic steels AISI 304L and AISI 316, the ferritic steel Sea Cure-1 and the duplex steel 2205. Table 1 shows the compositions of these materials. Working electrodes of different steels studied were obtained (area exposed: 10 mm x 10 mm), and, before each test, the electrodes were polished with SiC paper of different granulometry up to # 1500, rinsed with deionized water, and dried by natural airflow.
Table 1: Chemical composition of the four stainless steels studied.
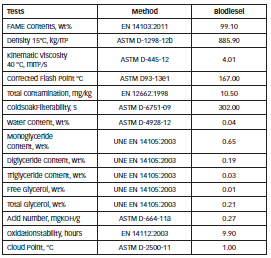
An electrochemical cell of Pyrex® glass with three electrodes (ASTM G-5), with a reference electrode of Ag/AgCl and platinum counter, was used for the electrochemical tests. The solution used for the electrochemical measurements was an industrial biodiesel produced from soy oil. The properties of this biodiesel were determined according to ASTM and EN standards and are shown in Table 2.
Table 2: Properties of the soy biodiesel used for the electrochemical measurements.
Due to the high resistivity of the biodiesel, an aqueous extraction was performed to obtain the aqueous ionic fraction of the biofuel, which generates the corrosion process. A quantity of 150 ml of biodiesel and 300 ml of deionized water were measured, mixed for 10 minutes to form an emulsion, and allowed to stand for 20 hours in a separating funnel. After elapsed, the aqueous phase was used as a medium for electrochemical assays [22]. The tests were conducted in a naturally aerated environment and at room temperature (25-30ºC).
The corrosion rates were measured by three different methods and the results were compared. On the one hand, electrochemical techniques based on the Stern-Geary equation were used, calculating the polarization resistance, Rp for the method of Linear Polarization and Electrochemical Impedance Spectroscopy (EIS), and on the other hand, a Gravimetric Test was carried out. The Stern-Geary equation [25] relates the density of corrosion current, icorr, with Rp, for simple reactions, controlled by the charge transfer (Eq. 1).
![]()
Where ba and bc are the anodic and cathodic Tafel slopes, respectively. To determine the Tafel slopes, potentiodynamic polarization curves were obtained in a reduced potential range of ± 30 mV of the corrosion potential Ecorr. According to the Linear Polarization Method [25], Rp can be obtained graphically as the tangent to the curve Ecorr (Eq. 2).
![]()
Measurements of EIS were also made with different previous immersion times (0, 1, 3 and 5 hours), using Gamry® Reference 300 potentiostat/galvanostat with an initial period of system stabilization of 600 seconds to determine the open circuit potential, Eoc, and a potential amplitude of 10 mV around this potential. To adjust the results, the method of nonlinear least squares designed by Bouckamp was used. According to the EIS technique [25], Rp values were obtained from the Nyquist plots, considering the following definition (Eq. 3).
![]()
where Z´ represents the real part of the complete faradaic impedance. Applying Faraday’s law (Eq. 4) [26], the corrosion current density, icorr, became the rate of penetration of the material, Vcorr expressed in mpy.

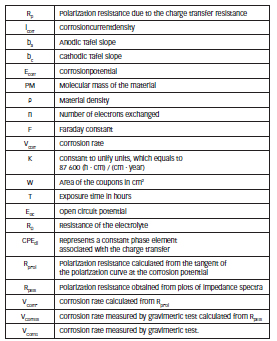
Where PM is the molecular mass of the material, n is the number of electrons exchanged, ρ is the material density and F is the Faraday constant.
Gravimetric tests were performed following the ASTM G31-72 standard norm. The test samples of AISI 304L (width: 2.61, length: 5.04 cm and thickness 0.30 cm), AISI 316 (width: 2.54 cm, length: 4.94 cm and thickness: 0.30 cm), Sea Cure-1 (width: 2.50 cm, length: 4,98 cm and thickness: 0.06 cm) and 2205 (width: 2.48 cm, length: 5.00 cm and thickness: 0.14 cm) were grounded with SiC paper up to #1500, and then were degreased with demineralized water and detergent, rinsed with alcohol and dried with airflow. The dried specimens were weighed on analytical balance with an accuracy of 0.0001 g. For immersion, the samples were clamped with plastic tweezers and placed inside glass containers containing pure biodiesel, ensuring that the coupons remained completely submerged in the medium. Special care was taken to ensure that the metal springs of the tweezers had no contact with the samples or biodiesel.
Figure 1 shows the coupons immersed in biodiesel. After 383 days (9192 hours) of exposure, the samples were extracted and their appearance regarding deposits, corrosion products or pit presence was examined. Demineralized water and detergent were used to remove fatty material, and then the coupons were placed in beakers containing alcohol to carry out ultrasound for 5 minutes to eliminate products that could be attached to the surface affecting the final weight measurement. The coupons were then dried with airflow and weighed again on the analytical balance to determine the weight change. The corrosion rate was calculated from (Eq. 5) [11,17]:
![]()
where K is a constant to unify units, which equals to 87 600 (h ∙ cm) / (cm ∙ year), W is the area of the coupons in cm2, T is the exposure time in hours and ρ is the density of material in g/cm3.

Figure 1: Steel coupons arranged for the immersion test in soy biodiesel.
Results and Discussion
The potentiodynamic curves obtained for the four steels studied in biodiesel were very similar (Figure 2). The materials maintained their passivity to very high values of potential. Steel 2205 presented the noblest Ecorr value, equal to - 37 mV. This result is consistent with the expected behavior against corrosion for steel compared to other duplex steels. Because duplex stainless steels have a lower density of nonmetallic inclusions and secondary phases than single-phase stainless steels, it can be expected that they have higher resistance to corrosion than austenitic or ferritic steels [27-29].
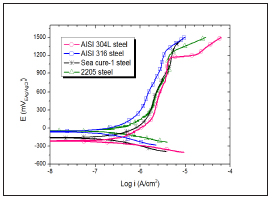
Figure 2: Potentiodynamic curves of the four steels studied in the biodiesel extract.
Figure 3 shows the evolution of Eoc versus time for the materials studied. It is observed that the four alloys are stabilized, although they require different times for this. Steels AISI 316 and 2205 are the first to achieve stable potentials during the first 2 hours. Austenitic steel 304L, requires approximately 3 hours to stabilize. Although steel Cure-1, among the four materials, shows the least favorable response, the stability of the potential reached after 5 hours indicates a good behavior against corrosion in this medium. According to Hodges, a ferritic alloy of high purity has outstanding resistance to attack in a wide variety of media containing chloride ion and organic acids [30].
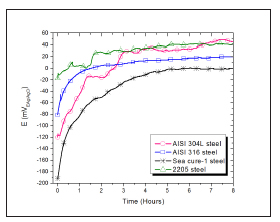
Figure 3: Evolution of the potential over time of the four steels studied in the biodiesel extract.
Table 3 shows the Ecorr for the steels studied in the biodiesel extract, as well as the values of Eoc at which the samples were stabilized after 8 hours of exposure after 8 hours.
Table 3: Ecorr and Eoc potentials.
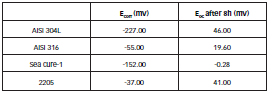
Once the balance was achieved, these potentials remained constant over time, and there was no sign of localized attack, so it can be deduced that the four stainless steels studied are resistant to pitting in the biodiesel extract studied [27].
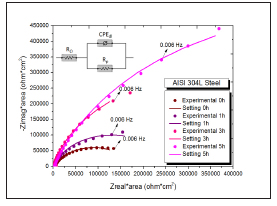
Figure 4: shows the Nyquist plots for different immersion times until 5 hours, and the enlargement of the capacitive arcs with increasing immersion time. The equivalent circuit used to adjust the impedance results obtained for the four materials at different immersion times is shown in the insert of Figure 4. This circuit is usually used to describe the behavior of stainless steels in different media, indicating the physical meaning of the elements [15,31-33]. RΩ corresponds to the resistance of the electrolyte, Rp is the polarization resistance due to the charge transfer resistance and CPEdl represents a constant phase element associated with the charge transfer.
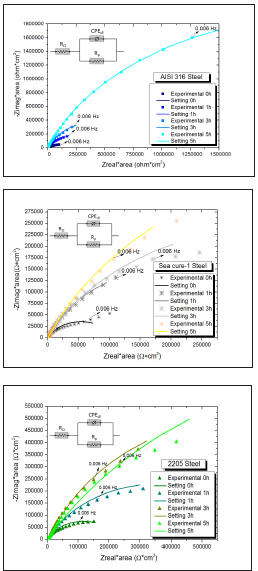
Figure 5: Nyquist plots of the four steels studied in biodiesel for different immersion times. a) AISI 304L Steel, b) AISI 316 Steel, c) Sea cure-1 Steel, d) 2205 Steel.
The increase in polarization resistances Rp, which would result in a decrease in corrosion currents [24], is shown in Figure 5. This would indicate an increase in corrosion resistance over time [20], matching with that observed in Figure 3 about the evolution of the open circuit with time.
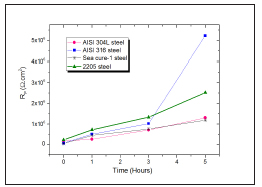
Figure 6: Rp measured at different immersion times.
Table 4 shows the values of Tafel slopes, the calculated icorr values, and the Rp values without immersion, obtained by the two electrochemical techniques. These parameters were used to calculate the corrosion rates by the Stern-Geary equation [25]. RpPol corresponds to the polarization resistance calculated from the tangent of the polarization curve at the corrosion potential. RpEIS is the polarization resistance obtained from plots of impedance spectra. It is observed that the Rp values obtained by both methods show good agreement.
Table 4: Values of Tafel slopes and icorr values. Rp values obtained by the two methods (Linear Polarization and Electrochemical Impedance Spectroscopy).
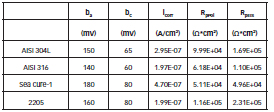
The values of icorr and Rp coincide with those presented by Deyab & Keera [34], when evaluating the corrosion behavior of carbon steel in biodiesel, obtained from used cooking oil, using electrochemical techniques.
Table 5 shows the values of corrosion rate measured for the four materials by the three techniques used. VCorrP is the corrosion rate calculated from RpPol, VCorrEIS was calculated from RpEIS and VCorrG is the corrosion rate measured by gravimetric test.
Table 5: Corrosion rates obtained by the three methods (Linear Polarization and Electrochemical Impedance Spectroscopy and Gravimetric).
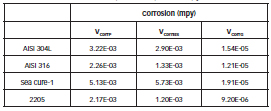
The duplex stainless steel 2205 showed the lowest corrosion rate, whereas the ferritic stainless steel Sea cure- 1 showed the highest corrosion rate. Austenitic stainless steels showed intermediate values of Vcorr. The fact that the sequence of velocity values from lower to higher was fulfilled for the four materials in the three methods indicates good agreement between the methods.
The velocities measured by the gravimetric method were markedly lower than those obtained by electrochemical methods. It is important to remember that electrochemical methods provide an instantaneous velocity value. Considering the results of the electrochemical tests, which showed increased resistance to corrosion of materials in the first 8 hours of study, it is expected that the corrosion rate obtained in weight loss tests, which is a speed averaged over time, is lower. It should also be noted that electrochemical methods involving disturbance of the system with an external signal could result in a higher value of corrosion rate [35].
The four materials showed very low corrosion rates, in the order of 1 micron/year by electrochemical methods, and even lower by gravimetric tests.
For stainless steel Q235evaluated in rapeseed biodiesel at 43 °C for 60 days, E. Hu et al.23 found a corrosion rate of 1.50E-03mpy, a value similar to the instantaneous velocities. In contrast, working with stainless steels 304, 444 and 439 for a time period similar to that tested in the gravimetric tests in this study (300 days), E. Miranda [19] found velocities in the order of 10-6mpy, similar to the time-averaged velocities, VCorrG. For steel AISI 316 in palm biodiesel and working at 80 °C, M. Fazal et al. [11] found a corrosion rate of 1.15E-2 mpy. According to these authors [36], it is expected that this velocity value is greater than those presented in Table 6, because the values were obtained working at a higher temperature.
There is evidence of diversity in the results of corrosion rates of ferrous materials in different types of biodiesel. In the literature it is reported that the compatibility of a material and the biodiesel with which it is in contact, will depend on the origin of the biofuel [4,9,18,37]. This may be explained by the fact that some authors consider the presence of short chain organic acids as responsible for increasing the corrosivity of biodiesel.
The appearance of these acids is due to the oxidation of the unsaturated fatty acids contained in biodiesel [1,3,14]. The amount and type of saturated and unsaturated fatty acids that biodiesel will contain will depend on the raw material used for its production [8].
In future works the evaluation of the corrosion rates of these materials in biodiesel, with different contents of unsaturated fatty acids, will be carried out to obtain more precise information in this respect.
Conclusions
The four stainless steels studied have good corrosion resistance and can therefore be used in contact with soy biodiesel. The materials behaved similarly in the medium studied, with low rates of corrosion, with duplex steel2205 showing the lowest corrosion rate with the three methods used.
The comparison of the three methods to measure corrosion rates indicates good agreement between the results of electrochemical methods. Compared with the values obtained with the gravimetric method; the instantaneous velocities were higher and can be used as a safe value for this design. The application of electrochemical techniques to determine the corrosion rates in the biodiesel extract was satisfactory, because they have the advantage of reduced test time. These results can be supplemented by other electrochemical tests, such as measurement of open circuit potential over time or EIS measurements with different immersion times, to obtain an approximation of the kinetic behavior of the electrochemical process.
List of Symbols
Acknowledgements
We thank the National Agency for Scientific and Technological Promotion by Grant No. PICT-2012-2952, the National Council of Scientific and Technical Research (CONICET) and the BIONOGOYÁ Company, who donated the biodiesel used in the present research.
1. Baum A. Stress, intrusive imagery, and chronic distress. Heal. Psychol. 9(6): 653–675, 1990.
2. Milazzo, M. F., Spina, F., Cavallaro, S. and Bart, J. C. J. “Sustainable soy biodiesel”. Renew. Sustain. Energy Rev. 27: 806–852, 2013.
3. Elshaib, A. A., Kamal, M. M. and Elahwany, A. A. “Performance of a diesel engine fueled by waste cooking oil biodiesel”, J. Energy Inst., 87: 11–17, 2014.
4. Prakash, R., Singh, R. K. and Murugan, S. “Experimental studies on combustion, performance and emission characteristics of diesel engine using different biodiesel bio oil emulsions”, J. Energy Inst. 88: 64–75, 2015.
5. Pérez-Quiroz, J. T. et al. “Corrosión y degradación de materiales por bio- combustibles”, OmniaScience, 69–85, 2013.
6. Dhar, A. and Agarwal, A. K. “Effect of Karanja biodiesel blend on engine wear in a diesel engine”, Fuel 134: 81–89, 2014.
7. Rashedul, H. K. et al.“The effect of additives on properties, performance and emission of biodiesel fuelled compression ignition engine”, Energy Convers. Manag. 88: 348–364, 2014.
8. Mofijur, M. et al. “Effect of biodiesel from various feedstocks on combustion characteristics, engine durability and materials compatibility: A review”, Renew. Sustain. Energy Rev. 28: 441–455, 2013.
9. Sorate, K. A. and Bhale, P. V. “Biodiesel properties and automotive system compatibility issues”, Renew. Sustain. Energy Rev. 41: 777–798, 2014.
10. Maru, M. M. et al. “Biodiesel compatibility with carbon steel and HDPE parts”, Fuel Process. Technol. 90: 1175–1182, 2009.
11. Fazal, M. A., Haseeb, A. S. M. A. and Masjuki, H. H. “Degradation of automotive materials in palm biodiesel”, Energy 40: 76–83, 2012.
12. Fazal, M. A., Haseeb, A. S. M. A. and Masjuki, H. H. “Comparative corrosive characteristics of petroleum diesel and palm biodiesel for automotive materials”, Fuel Process. Technol. 91: 1308–1315, 2010.
13. Jin, D., Zhou, X., Wu, P., Jiang, L. and Ge, H. “Corrosion behavior of ASTM 1045 mild steel in palm biodiesel”, Renew. Energy 81: 457–463, 2015.
14. Singh, B., Korstad, J. and Sharma, Y. C. “A critical review on corrosion of compression ignition (CI) engine parts by biodiesel and biodiesel blends and its inhibition”; Renew. Sustain. Energy Rev. 16: 3401–3408, 2012.
15. Aseeb, A. S. M. A., Fazal, M. A., Jahirul, M. I. and Masjuki, H. H. “Compatibility of automotive materials in biodiesel: A review”, Fuel 90: 922–931, 2011.
16. Kerner, Z., Horvath, A. and Nagy, G. “Comparative electrochemical study of 08H18N10T , AISI 304 and AISI 316L stainless steels”, Electrochim. Acta 52: 7529– 7537, 2007.
17. Fernandes, J. C. S. and Ferreira, M. G. S. “Electrochemical impedance studies on pure aluminium in carbonate solution”, J. Appl. Electrochem. 20: 874–876, 1990.
18. Cursaru, D. L., Branoiu, G., Ramadan, I. and Miculescu, F. “Degradation of automotive materials upon exposure to sunflower biodiesel”, Industrial Crops and Products 54: 149–158, 2014.
19. Kovács, A., Tóth, J., Isaák, G. and Keresztényi, I. “Aspects of storage and corrosion characteristics of biodiesel”, Fuel Process. Technol. 134: 59–64, 2015.
20. Miranda, E. F. D. E. “Comportamento quanto à corrosão de aços inoxidaveis na produção do biodiesel”, Escola de Engenharia Mauá do Centro Universitário do Instituto Mauá de Tecnologia, 2009.
21. Román, A. S., Barrientos, M. S., Harms, F., Méndez, C. M. and Ares, A. E. “Resistencia corrosión de acero inoxidable AISI 304L en biodiesel de soja”, An. AFA 27; 14–18, 2016.
22. Román, A. S., Mendez, C. M. and Ares, A. E. “Corrosión de un acero inoxidable austenítico en biodiesel”, An. AFA 26: 128–134, 2015.
23. Román, A. S., Méndez, C. M. and Ares, A. E. “Corrosion Resistance of Stainless Steels in Biodiesel” in Shape Casting: 6th International Symposium TMS (The Minerals, Metals & Materials Society), 109–116, 2016.
24. Hu, E., Xu, Y., Hu, X., Pan, L. and Jiang, S. “Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol”, Renew. Energy 37: 371–378, 2012.
25. Valcarce, M. B., Vázquez, M. and Sánchez, R. S. “Determinación de la velocidad de corrosión de latón al aluminio en agua potable”, in CONGRESO CONAMET/SAM 2004, 1–6, 2004.
26. Lorenz, W. J. “Determination of corrosion rates by electrochemical DC and AC methods”, Corros. Sci. 21: 647–672, 1981.
27. Galvele, J. R. and Duffó, G. S. “Degradación de materiales. Corrosión”, 2006.
28. Méndez, C. M. and Ruiz, E. R. “Evaluación de aceros inoxidables dúplex para la construcción de un digestor”. Rev. Cienc. y Tecnol. 16; 44–48, 2011.
29. Gojkovic, S. L., Zecevic, S. K., Obradovic, M. D. and Drazic, D. M. “Oxygen reduction on a duplex stainless steel”, Corros. Sci. 40: 849–860, 1998.
30. Deyab, M. A. and Keera, S. T. “Cyclic voltammetric studies of carbon steel corrosion in chloride-formation water solution and effect of some inorganic salts”, Egypt. J. Pet. 21, 31–36, 2012.
31. Hodges, R. J., Schwartz, C. D. and Gregory, E. “Corrosion Resistance of an Electron Beam Refined 26%Cr-1%Mo Ferritic Stainless Steel”, Br. Corros. J. 7: 69–75, 1972.
32. Boudalia, M. et al. “Corrosion Inhibition of Organic Oil Extract of Leaves Of Lanvandula Stoekas on Stainless Steel in Concentrated Phosphoric Acid Solution”, Int. J. Electrochem. Sci. 8: 7414–7424, 2013.
33. Huang, C., Chang, Y. and Chen, S. C. “The electrochemical behavior of austenitic stainless steel with different degrees of sensitization in the transpassive potential region in 1 M H2SO4 containing chloride”, Corros. Sci. 46: 1501–1513, 2004.
34. Kocijan, A., Kek, D. and Jenko, M. “The corrosion behaviour of austenitic and duplex stainless steels in artificial saliva with the addition of fluoride”, Corros. Sci. 53: 776–783, 2011.
35. Deyab, M. A. and Keera, S. T. “On corrosion and corrosion inhibition of carbon steel in stored biodiesel : electrochemical (AC and DC) studies”, J. Taiwan Inst. Chem. Eng. 68, 187–191, 2016.
36. Carranza, R. “Técnicas electroquímicas para la determinación de la velocidad de corrosión”, Instituto Sábato. UNSAM-CNEA. 2009.
37. Haseeb, A. S. M. A., Masjuki, H. H., Ann, L. J. and Fazal, M. A. “Corrosion characteristics of copper and leaded bronze in palm biodiesel”, Fuel Process. Technol. 91: 329–334, 2010.
38. Kaul, S. et al. “Corrosion behaviour of biodiesel from seed oils of Indian origin on diesel engine parts”, Fuel Process. Technol. 88: 303–307, 2007.
Recibido: 15/12/2017.
Aprobado: 05/04/2018.














