Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Odontológica Latinoamericana
versão On-line ISSN 1852-4834
Acta odontol. latinoam. vol.22 no.3 Buenos Aires dez. 2009
ARTÍCULOS ORIGINALES
The use of topical subgingival gels of non-steroidal anti-inflammatory drugs (NSAIDs) as an adjunct to non-surgical management of chronic periodontitis
Esteban R. Funosas1,2, Livia Escovich3, Lorella Maestri2
1 Department of Periodontology,
2 Pharmacology and
3 Oral Medicine, Faculty of Dentistry, University of Rosario, Rosario, Argentina
CORRESPONDENCE Dr. Esteban Funosas Catedra de Periodoncia. Facultad de Odontologia Universidad Nacional de Rosario Santa Fe 3160, 7o piso (2000) Rosario Provincia de Santa Fe, Argentina e-mail: efunosas@hotmail.com
ABSTRACT
The treatment of chronic periodontitis consists of conventional mechanical debridement and removal of plaque and calculus1. Topical NSAIDs could be used to complement the therapy as an adjunct to resolve the inflammatory process and clinical signs of the disease more rapidly. A randomized clinical trial was performed on 33 systemically healthy patients diagnosed with chronic periodontitis, aged 21 to 40 years. All patients underwent scaling and root planing on one quadrant per week, and sub-gingival gel was applied 48 hours after each session. Patients were grouped into 4 treatment protocols with gels containing: (1) placebo, (2) 1% acetyl-salicylic acid (ASA), (3) 1% ketoprofen (KTP) and (4) 2% ketoprofen. The clinical variables studied were: probing depth, attachment level, tooth mobility, plaque index, gingival index and bleeding on probing. All protocols (groups 1, 2, 3 and 4) induced a reduction of probing depths, plaque and gingival indices and bleeding on probing. The 1% ASA and 2% KTP protocols (groups 2 and 4) significantly reduced the probing depth variable (ANOVA p<0.05).
Key words: NSAIDs; Chronic periodontitis; Topical administration.
RESUMEN
Topicacion subgingival con geles de drogas antiinflamatorias no esteroides (AINEs) como coadyuvante en el manejo no quirúrgico de la periodontitis crónica
El tratamiento de la periodontitis crónica, consiste en el desbridamiento convencional mecánico de remoción de placa y calculo1. El uso de AINEs en forma tópica como complemento de la terapia podría coadyuvar a resolver con mas rapidez el proceso inflamatorio y los signos clínicos de la enfermedad. Se realizo un estudio clínico randomizado en el que participaron 33 pacientes con diagnostico de periodontitis crónica, sistemicamente sanos de edades entre 21 y 40 anos. A todos los pacientes se les realizo raspaje y alisado radicular en un cuadrante por semana, aplicándose subgingivalmente el gel a las 48 hs de cada sesión. Para esto, los pacientes fueron agrupados en 4 protocolos de tratamiento con geles conteniendo: (1) placebo, (2) acido acetilsalicilico (AAS) 1%, (3) Ketoprofeno (KTP) 1% y (4) ketoprofeno 2%. Las variables clínicas estudiadas fueron: profundidad de sondaje, nivel de inserción, movilidad dentaria, índice de placa, índice gingival y sangrado al sondaje. Todos los protocolos (grupo 1, 2, 3 y 4) indujeron una disminución en la profundidad al sondaje, los índices de placa y gingival y el sangrado al sondaje. Los protocolos del AAS al 1% y el KTP 2% (grupo 2 y 4) redujeron significativamente la variable de profundidad al sondaje (ANOVA p<0,05).
Palabras clave: NSAIDs; Chronic periodontitis; Topical administration.
INTRODUCTION
According to the American Academy of Periodontology, chronic periodontitis is an infectious disease resulting in inflammation within the tooth support tissues and leading to the progressive loss of attachment and bone, and is characterized by gingival inflammation, pocket formation and/or gingival recession1. The anti-infectious therapy of periodontal disease includes mechanical, surgical and non-surgical treatment as well as the use of antibiotics and anti-inflammatory drugs administered systemically2-7 and topically8,9. There is currently sufficient evidence showing that both cyclooxygenase (COX) and lipoxygenase (LOX) products of arachidonic acid are involved in the pathogenesis of periodontal disease. Most research into the subject focuses on the COX pathway, specifically prostaglandins (PG). There are large quantities of PGs at inflamed sites, which is why the gingival crevicular fluid (GCF) is an ideal medium for monitoring changes during the periodontal healthdisease process. PGs are associated to tissue destruction, metabolic changes in the fibroblast and bone resorption10. High levels of prostaglandin E2 (PGE2) in GCF are correlated positively to periodontal inflammation and tissue destruction in humans11-18 and animals19,20, and many studies relate PGE2 levels to active periodontal disease21,22. In 1992 Abramson et al.23 showed that levels of prostaglandin E2 and Thromboxane B2 in human gingival fluid remained constant during administration of flurbiprofen in patients with adult early periodontitis. Negai et al.24 showed that prostaglandin E2 stimulates bone resorption. Other studies showed that arachidonate metabolites in the LOX pathway, specifically 12-HETE and 15-HETE, are mediators of tissue inflammation in patients with gingival disease and advanced periodontal disease respectively25-27.
The topical use of non-steroidal anti-inflammatory drugs (NSAIDs) to supplement periodontal therapy might help resolve the inflammatory process and the clinical signs of the disease sooner, because NSAIDs block PG synthesis by inhibiting the COX pathway in the arachidonic acid cascade. The aim of this study was to find a safe NSAID which could be applied topically and intracrevicularly as an adjunct to the treatment of chronic periodontal disease, to revert its clinical variables.
MATERIALS AND METHODS
Patients
Thirty-three (33) patients aged 21 to 40 years were included in this study, which was approved by the bioethics committee of Rosario National University, Argentina.
Inclusion criteria: systemically healthy patients diagnosed with chronic periodontitis with periodontal pockets ≥ 4 millimeters deep on at least three teeth per quadrant. The systemically healthy condition was determined by means of clinical history, physical examination and laboratory studies.
Exclusion criteria: patients with chronic diseases, smokers, patients who had taken any kind of medication during the previous thirty days.
Intracrevicular gel preparation
To prepare the 1% ASA gel, 4 g sodium carboxymethyl cellulose (a fine, creamy-white, aqueous flowable powder, insoluble in ethanol, acetone and chloroform) were added to 200 ml distilled water, shaken and left to rest, thus producing an opalescent gel with pH 8. Separately, we prepared the 0.1% solution of ASA previously dissolved in the minimum amount of alcohol, citric acid and sodium bicarbonate, in the minimum amount of water, as excess water would reduce gel viscosity. The pH value for this solution was 6. The solution was added to the gel at room temperature, producing agglutination, which was corrected by shaking hard under gentle heat in order not to break down the solution. The final pH value of the preparation was 8. The ketoprofen gel was prepared by adding 200 ml distilled water to 4 g sodium carboxymethyl cellulose, shaking and leaving to rest, thus producing an opalescent gel with pH 8. Separately, 2 g ketoprofen (for the 1% concentration) and 4 g ketoprofen (for the 2% concentration), citric acid and sodium bicarbonate were dissolved in the minimum amount of water and added to the previous solution under gentle heat and shaking to prevent agglutination. A placebo gel was prepared similarly, by adding 4 g carboxymethyl cellulose to 200 ml water. Pharmacological treatment protocols All patients received non-surgical periodontal treatment as follows: a scaling and root planing session on one mouth quadrant at seven-day intervals (4 consecutive weeks). Gel was applied intracrevicularly 48 hours after each session. Patients were divided into 4 experimental groups. Intracrevicular gel was applied to each group, delivered with syringes with 21Gauge x 11/2 0.80 x 40 needles after drying the area with a jet of air. Group 1: placebo gel (n=6)
Group 2: 1% ASA gel (n=9)
Group 3: 1% ketoprofen gel (n=8)
Group 4: 2% ketoprofen gel (n=10)
Clinical variables studied
The clinical indices were recorded before starting the scaling and planing (baseline) and at 30 days post-baseline (one week after the last gel application).
• Probing depth
Measured from the free gingival margin to the bottom of the pocket, with a standardized pressure of 25 to 30 g using a second generation probe with pressure controlled by Marquis type marking, Pro Dentec brand Type I.
• Attachment level
Determined from the cementoenamel junction to the bottom of the pocket with standardized pressure of 25 to 30 g using a second generation probe with pressure controlled by Marquis type marking, Pro Dentec brand Type I.
• Tooth mobility37.
Degree 1: 0.2 – 1 mm horizontal crown mobility Degree 2: more than 1 mm horizontal crown mobility Degree 3: horizontal and vertical crown mobility
• Plaque index37
• Gingival index37
• Bleeding on probing (Regardless of gingival index, and as a result of gentle probing) 0: Negative; 1: Positive.
Statistical analysis
Each protocol was compared statistically to its control group using Student’s T test. The results of all the protocols were compared using ANOVA with subsequent multiple comparisons using Dunnet’s test. In each case, a level of p<0.05 was considered significant.
RESULTS
All the protocols studied individually (groups 1, 2, 3 and 4) produced a significant reduction in probing depth, plaque index, gingival index and bleeding on probing (student t test p<0.05) (Table 1). Nevertheless, on comparing the differences between baseline and thirty days of all protocols to each other, it was found that probing depth decreased significantly in protocols using 1% ASA and 2% KTP (groups 2 and 4) and that ASA (group 2) was clinically the most effective in reducing it (Fig. 1). Gingival index, bleeding on probing and plaque index did not show significant differences for any of the groups (Table 2). None of the protocols significantly altered the gain in attachment level or the reduction in tooth mobility (Table 1).
Table 1: Effects of each protocol on the clinical variables studied 30 days post-baseline.
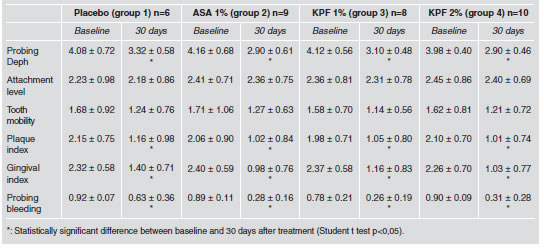
Table 2: Comparison of the reduction in the clinical variables induced by each protocol.
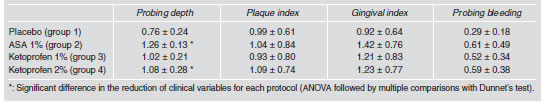
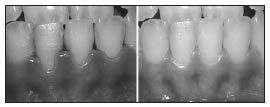
Fig. 1: Before treatment with 1% ASA (left) and after treatment with 1% ASA (right).
DISCUSSION
Until the nineteen-nineties, there were only a few studies on humans providing evidence of the effectiveness of NSAIDs on reducing the progression of periodontal disease. The first studies performed by Paolantonio et al.28 using subgingival irrigation with 1% ASA in patients with periodontitis reduced the subclinical inflammation of the periodontal pockets. Corry and Moran29 suggest that using strips of polymethacrylate cement as a delivery vehicle for sustained release of NSAIDs such as indometacin, tolmetin and mefenamic acid might be an important tool for treating periodontal diseases. Our studies showed that administering intracrevicular 1% ASA and 2% KTP gel as an adjunct to periodontal treatment in patients with chronic periodontitis can significantly reduce probing depth. We propose using 1% ASA and 2% KTP with bioadhesive gels as a delivery vehicle30 instead mouthrinses28,29,31,32, because gel pharmaceutical presentations have the advantage or remaining in situ longer than irrigants and the technique is simpler than manipulating polymethacrylate strips in the periodontal pockets. Moreover, an effective means for treating the inflammatory lesion, which is limited to the periodontal tissues, will reduce the gastrointestinal and renal adverse effects often produced by NSAIDs33,34.
None of our protocols altered the clinical attachment level or tooth mobility. Although there is evidence of the effectiveness of systemically administered NSAIDs on periodontal disease33 the studies are controversial regarding the alteration of attachment level. Del Puente et al.35 showed that patients suffering from adult periodontitis who were taking systemic NSAIDs suffered less periodontal attachment loss. In contrast, Heasman and Seymour36 report that systemic NSAIDs do not affect the amount of attachment loss. Since the reduction of attachment loss depends directly on the reduction of the inflammatory exudate in the crevicular liquid, it is logical to assume that a systemically administered NSAID, which remains for a longer time and at a higher concentration in the crevicular fluid than a topical NSAID does, should more effectively induce attachment gain. However, considering that clinical data on the effects of systemic administration of NSAIDs are not conclusive and have not been shown to be clinically superior to topical administration, it would be prudent to consider local administration in order to avoid adverse systemic effects.
The topically administered NSAID protocols used in this study did not alter the plaque index or gingival index more than the placebo did. As the infectious agents that produce and maintain the inflammation need to be reduced to enable NSAID anti-inflammatory action, the plaque index may be expected to be a variable independent from NSAID application, and more related to mechanical debridement, base therapy or changes in the patient’s brushing technique. Finally, although the base therapy is the conventional and unarguably the most effective treatment for chronic periodontitis, we believe that the use of adjuncts such as 1% ASA or 2% KTP administered topically by means of intracrevicular gel, as suggested, could aid the clinical reduction of probing depth in this disease. Thus, by changing the patient’s hygiene habits plus periodontal therapy, a mechanical anti-infectious action on bacterial plaque could be achieved, after which the anti-inflammatory action of NSAIDs could be used on a relatively germ-free terrain as an adjunct for tissue repair.
ACKNOWLEDGEMENT
The authors are grateful to Dr. Romina F. Aromando for her review of the manuscript.
1. Flemming TF. Periodontitis. Ann Periodontol 1999;4:32-37. [ Links ]
2. Williams R, Jeffcoat M, Howell T, et al. Three-year clinical trial of flurbiprofen treatment of human periodontitis: Preliminary analysis [Abstr.2062]. J Dent Res 1988; 67 (Spec. Issue):370. [ Links ]
3. Williams R, Jeffcoat M, Howell T et al. Three-year clinical trial of flurbiprofen treatment in humans: Post-treatment period [Abstr.1617]. J Dent Res 1991;70 (Spec. Issue):468. [ Links ]
4. Haesman PA, Ward A, Barrett AW, Seymour RA, Edwards G. Flurbiprofen in human crevicular fluid analyzed by high-performance liquid chromatography. J Periodont Res 1990;25:88-92. [ Links ]
5. Haesman PA, Offenbacher S, Collins JG, Edwards G, Seymour RA. Flurbiprofen in the prevention and treatment of experimental gingivitis. J Clin Periodontol 1993;20: 732-738. [ Links ]
6. Heasman PA, Seymour RA, Kelly PJ. The effect of systemically- administered flurbiprofen as an adjunct to toothbrushing on the resolution of experimental gingivitis. J Clin Periodontol 1994;21:166-170. [ Links ]
7. Taiyeb Ali TB, Waite IM. The effect of systemic ibuprofen on gingival inflammation in humans. J Clin Periodontol 1993;20:723-728. [ Links ]
8. Heasman PA, Benn DK, Kelly PJ et al. The use of topical flurbiprofen as an adjunct to non-surgical management of periodontal disease. J Clin Periodontol 1993;20:457-464. [ Links ]
9. Jeffcoat MK, Reddy MS, Haigh S et al. A comparison of topical ketorolac, systemic flurbiprofen, and placebo for the inhibition of bone loss in adult periodontitis. J Periodontol 1995;66:329-338. [ Links ]
10. Kinane DF. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontology 2000 2000;24:215-225. [ Links ]
11. Abramson MM, Wolff LF, Offenbacher S, Aeppli DM, Hardie ND, Friedman HM. Flurbiprofen effect on gingival crevicular fluid prostaglandin and thromboxane levels in humans. J Periodontal Res 1992;275:539-543. [ Links ]
12. Damare SM, Wells S, Offenbacher S. Eicosanoids in periodontal diseases: potential for systemic involvement. Adv Exp Med Biol 1997;433:23-35. [ Links ]
13. Dewhirst FE, Moss DE, Offenbacher S, Goodson JM. Levels of prostaglandin E2, thromboxane, and prostacyclin in periodontal tissues. J Periodontal Res 1983;182: 156-163. [ Links ]
14. Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1 beta, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor alpha in experimental gingivitis in humans. J Periodontal Res 1993;284:241-247. [ Links ]
15. Offenbacher S, Odle BM, Green MD et al. Inhibition of human periodontal prostaglandin E2 synthesis with selected agents. Agents Actions 1990;293:232-238. [ Links ]
16. Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res 1986;212: 101-112. [ Links ]
17. Offenbacher S, Odle BM, Gray RC, Van Dyke TE. Crevicular fluid prostaglandin E levels as a measure of the periodontal disease status of adult and juvenile periodontitis patients. J Periodontal Res 1984;191:1-13. [ Links ]
18. Offenbacher S, Farr DH, Goodson JM. Measurement of prostaglandin E in crevicular fluid. J Clin Periodontol 1981; 84:359-367. [ Links ]
19. Offenbacher S, Williams RC, Jeffcoat MK et al. Effects of NSAIDs on beagle crevicular cyclooxygenase metabolites and periodontal bone loss. J Periodontal Res 1992; 273:207-213. [ Links ]
20. Offenbacher S, Odle BM, Braswell LD et al. Changes in cyclooxygenase metabolites in experimental periodontitis in Macaca mulatta. J Periodontal Res 1989;241:63-74. [ Links ]
21. Salvi GE, Brown CE, Fujihashi K et al. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res 1998;334:212-225. [ Links ]
22. Goodson JM, Dewhirst FE, Brunetti A. Prostaglandin E2 levels and human periodontal disease. Prostaglandins 1974; 61:81-85. [ Links ]
23. Abramson MM, Wolff LF, Offenbacher S, Aeppli DM, Hardie ND, Friedman HM. Flurbiprofen effect on gingival crevicular fluid prostaglandin and tromboxane levels in humans. J Periodont Res 1992;27:539-543. [ Links ]
24. Nagai M, Suzuki Y, Ota M. Systematic assessment of bone resorption, collagen synthesis, and calcification in chick embryonic calvaria in vitro: Effects of prostaglandin E2. Bone 1993;14:655-659. [ Links ]
25. El Attar T, Lin H, Killoy W, Vanderhoek J., Goodson J. Hydroxy fatty acids prostaglandin formation in diseased human periodontal pocket tissue. J Periodont Res 1986; 21:169-176. [ Links ]
26. Sidhagen B, Hamberg M, Fredholm B. Formation of 12Lhydroxyeicosatetraenoic acid (12-HETE) by gingival tissue. J Dent Res 1982; 61:761-763. [ Links ]
27. El Attar T, Lin H. Relative conversion of arachidonic acid through lipoxygenase and cyclooxygenase pathways by homogenates of diseased periodontal tissues. J Oral Pathol 1983;12. [ Links ]
28. Paolantonio M; Fanali S; Di Genova S. Effetto di irrigazioni subgengivali con acetilsalicilato sul numero di leucociti polimorfonucleati in tasche parodontali di media profondita. Minerva Stomatol 1995;44:265-271. [ Links ]
29. Corry D, Moran J. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-ste roidal anti-inflammatory drugs. Biomaterials 1998;19:1295-1301. [ Links ]
30. Needleman IG, Smales FC, Martin GP. An investigation of bioadhesion for periodontal and oral mucosal drug delivery. J Clin Periodontol 1997;24(6):394-400. [ Links ]
31. Preshaw PM, Lauffart B, Brown P, Zak E, Heasman PA. Effects of ketorolac tromethamine mouthrinse (0.1%) on crevicular fluid prostaglandin E2 concentrations in untreated chronic periodontitis. J Periodontol 1998;69(7): 777-783. [ Links ]
32. Okuda K, Adachi M, Iijima K. The efficacy of antimicrobial mouthrinses in oral health care. Bull Tokyo Dent Coll 1998;39(1):7-14. [ Links ]
33. Paquette DW, Lawrence HP, McCombs GB et al. Pharmacodynamic effects of ketoprofen on crevicular fluid prostanoids in adult periodontitis. J Clin Periodontol 2000; 27:558-566. [ Links ]
34. Nguyen AM, Graham DY, Gage T, Griffiths GR. Nonsteroidal anti-inflammatory drug use in dentistry: gastrointestinal implications. Gen Dent 1999;47(6):590-596. [ Links ]
35. Del Puente A, Shlossman M, Arevalo A, Knowler W, Genco R. Relationship of rheumatoid arthritis and periodontal disease [Abstr.2064]. J Dent Res 1988;67 (Spec. Issue):371. [ Links ]
36. Heasman PA, Seymour RA. An association between longterm non-steroidal anti-inflammatory drug therapy and the severity of periodontal disease. J Clin Periodontol 1990; 17:654-658. [ Links ]
37. Bascones Martinez A. Periodoncia. Cap 8. Ed. Ruan S.A. 1986. [ Links ]














