Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta Odontológica Latinoamericana
versão On-line ISSN 1852-4834
Acta odontol. latinoam. vol.24 no.2 Buenos Aires set. 2011
ARTÍCULOS ORIGINALES
Prevalence of Candida species in necrotic pulp with chronic periapical processes
Natalia Nastri1, Maria Nastri1, Virginia Jewtuchowicz1,2, Maria Mujica2, Cristina Iovanniti2, Ariel Gualtieri3, Jose Ponton4, Alcira Rosa1
1 Department of Microbiology. and Parasitology, Faculty of Dentistry, University of Buenos Aires, Argentina.
2 Department of Microbiology, Faculty of Medicine, University of Buenos Aires, Argentina.
3 Department of Biophysics, Faculty of Dentistry, University of Buenos Aires, Argentina.
4 Department of Immunology, Microbiology and Parasitology, School of Medicine and Odontology University of Basque Country, Leoia, Spain.
CORRESPONDENCE Dra. Alcira Cristina Rosa Catedra de Microbiologia y Parasitologia, Facultad de Odontologia, Universidad de Buenos Aires M.T. de Alvear 2142 (C1122AAH) Buenos Aires, Argentina. E-mail: flianastri@yahoo.com
ABSTRACT
The aim of this study was to identify species of the genus Candida in mucosa of oral cavity and in single-rooted teeth with pulp necrosis with chronic endodontic periapical processes, with radiographic images 2}4 mm and without clinical symptomatology, in immunocompetent patients. The study included 82 immunocompetent patients of both sexes aged 18-70 years with a clinical dental diagnosis of septic pulp necrosis. Samples were taken from root canals with sterile # 25 paper points and from oral mucosa with a sterile swab. Seven different Candida species were identified (C. albicans, C. dubliniensis, C. guilliermondii, C. krusei, C. parapsilopsis, C. tropicalis and C. glabrata). All of them were present in oral mucosa, while two of them (C. parapsilopsis and C. glabrata) were not identified in the periapical zone of necrotic canals. Considering all the samples isolated from oral mucosa, there was a significantly greater frequency of C. albicans than there was in the periapical zone of necrotic canals.
Key words: Candida; Dental Pulp Necroses.
RESUMEN
Prevalencia de especies de Candida en pulpas necróticas con procesos periapicales crónicos
El proposito de este estudio fue identificar especies del genero Candida en mucosa de cavidad bucal y dientes unirradiculares con necrosis pulpar con procesos periapicales cronicos de origen endodontico con imagenes radiograficas entre 2}4 mm sin sintomatologia clinica en pacientes inmunocompetentes. El estudio incluyo 82 pacientes inmunocompetentes de ambos sexos con edades entre 18-70 anos con diagnostico clinico dentario de necrosis pulpar septica. Las muestras fueron obtenidas del conducto radicular con conos de papel esteril # 25 y de la mucosa bucal mediante hisopo esteril. Se identificaron 7 especies diferentes de Candida (C. albicans, C. dubliniensis, C. guilliermondii, C. krusei, C. parapsilopsis, C. tropicalis y C. glabrata). Todas estuvieron presentes en mucosa bucal, mientras que dos de ellas (C. parapsilopsis y C. glabrata) no se identificaron en zona periapical de conductos con necrosis Del total de las muestras aisladas de mucosa bucal hubo una frecuencia significativamente mayor de C. albicans que la proporcion de estas levaduras en la zona periapical en conductos con necrosis.
Palabras clave: Candida; Necrosis de la pulpa dental.
INTRODUCTION
Microorganisms from reservoirs in the oral cavity can spread and act as a source of infection. When the dentin-pulp complex is infected, microorganisms invade and may cause disease in the pulp and in root canal system1-4. Most of those microorganisms are opportunistic pathogens. Their survival is favored by different environmental factors in the root canal (oxygen tension, redox potential, pH, temperature and type of nutrients), which may be responsible for selecting and establishing the dominant microbe species5-7.
Most pathogenic responses are related to the presence of microorganisms that have numerous virulence factors (microbial capsule, fimbriae, pili, lipopolysaccharides, enzymes, fatty acids, ammonia, etc.) 8-11. Microorganisms in the root canal system have been found to be capable of invading tubules in dentin walls down to depths of 150-250 μm, where they cannot be eliminated by the irrigants or medication used during root canal treatment12-15. Infection of the dentin-pulp complex may occur either as a result of necrosis produced by microorganisms or because microorganisms colonize necrotic tissue16-18.
Certain microorganisms may spread hematogenously from chronic apical and periapical processes to heart valves, prosthetic devices and other metastatic foci17,18. The aim of this work was to identify species of the genus Candida in samples from oral mucosa and chronic periapical processes of endodontic origin without clinical symptomatology in immunocompetent patients.
MATERIALS AND METHODS
Study population
The study was performed on samples isolated from 82 immunocompetent adults of both sexes aged 18 to 70 years; average age 43.3 ± 15.4, who visited the Faculty of Dentistry offices at the University of Buenos Aires in 2005-2009. Their evaluation included dental/medical history, clinical/radiographic examination and pulp vitality tests. The teeth selected were upper incisors diagnosed with pulp necrosis, with periapical lesion in chronic evolution (TROPE classification)19. Each tooth had crown-root integrity, with a 2 ± 4 mm circumscribed or diffuse radiolucent, radiographically visible periapical lesion without clinical symptomatology. Patients signed an informed consent form after the aim of the study was explained.
Exclusion criteria
Patients who were pregnant, had severe periodontal disease, systemic disease or had taken antibiotics, non-steroidal anti-inflammatory drugs and/or corticoids or anti-fungal medication. Permanent teeth with immature apex, teeth with difficult access to foramen, endodontic re-treatments or teeth with restorations.
Sampling
Patients rinsed their mouths with sterile distilled water, after which samples were taken from:
1. Oral mucosa, by swabbing the dorsal and ventral part of the tongue after relative isolation of the area with cotton rolls and aspiration with highpower suction.
2. Root canal: the tooth was completely isolated with a rubber dike and aspiration using highpower suction. A 10% povidone iodine solution was applied to the operating field, an opening made with a stone bur followed by sterile round drill #2, and a Maillefer/ Dentsply® K-file #15 was used to perform the catheterization and confirm that the canal could be reached. A # 25 paper point (Meta Biomed Co, LTD®) was inserted into the root canal and left for 1 minute. Then the paper point was suspended in a tube containing 1000 microliters of PBS transport medium (buffered solution of sterile Phosphate Buffer 0.1 M pH 7.4, kept at 4°C), which was vortexed and then processed.
Microbiological method
1. Direct microscopy studies of the samples, fresh and Gram-and Giemsa-stained20.
2. Cultures – samples were placed in selective and differential media. In order to isolate the yeast species, 100 μl microliters (0.1 ml) of the sample was transferred to a Petri dish containing solid chromogenic differential medium (CHROMagar® Candida, Paris, France). They were incubated at 30.C for 72 hs, and checked daily for growth.
The isolated yeasts were identified according to (A) the color developed in the chromogenic medium, (B) development of pseudomycelium with chlamydoconidia in 1%-Tween 80 milk agar (Jitaurong et al. 1993) and (C) carbohydrate assimilation profile using API ID 32D commercial systems (BioMérieux ®, France)20. Any green colored colony found on the CHROMagar Candida was subject to the following additional studies in order to complete the presumptive identification as C. dubliniensis: (A) D-xylose assimilation, (B) growth at 45.C (negative for dubliniensis) and (C) chlamydoconidia formation on Staib medium (Staib 1999) (Sullivan and Coleman, 1998; Jewtuchowicz, 2007). It was inoculated by stabbing on a sterile slide containing 3 ml of the medium, and incubated at 28.C for 48 hs in a moist chamber. Presence of chlamydoconidia was observed under microscope at 200X and 400X. Enzymatic activity (lipase) was visible on the 1% tween-80 and Ca chloride medium by the opaque precipitation zone.
Statistical analysis
The Candida species identified in the oral mucosa and canals with pulp necrosis were distributed according to frequencies. The Chi-square test for comparing frequencies in contingency tables was used, with Yates correction for continuity (significance level α =0.05), using PASW Statistics 18 (SPSS Inc.) software.
RESULTS
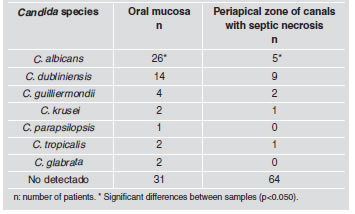
Seven Candida species were identified (C. albicans, C. dubliniensis, C. guilliermondii, C. krusei, C. parapsilopsis, C. tropicalis and C. glabrata). They were all identified from oral mucosa simples, while two of them (C. parapsilopsis and C. glabrata) were not found in the periapical zone of root canals with pulp necrosis (Fig. 1 and Fig. 2).

Fig. 1: Frequency distribution of Candida species identified in oral mucosa.

Fig. 2: Frequency distribution of Candida species in the periapical zone of canals with septic necrosis.
The difference between the two groups proved to be significant for C. albicans, which was much higher from oral mucosa samples than from the apical zone of canals with septic necrosis (p<0.050). For the rest of the species studied there were differences between the two areas, although they were not statistically significant (C. dubliniensis: p=0.368; C. guillier mondii: p=0.677; C. krusei: p≈1.000; C. parapsilopsis: p≈1.000; C. tropicalis: p≈1.000; C. glabrata: p=0.477 (Table 1).
Table 1. Presence of Candida species in oral mucosa and periapical zone of canals with septic necrosis.
DISCUSSION
In 2002, Nasia21 proposed that the true role of biofilms had not yet been elucidated, and that in the etiology of endodontic disease, mutation of microorganisms in biofilms brings about changes such as increased resistance to antimicrobial agents, increased adhesion, morphological and other changes which differ from the planktonic forms. As a result of this resistance, microorganisms in the biofilm may become reservoirs for the establishment of chronic infections. As a result of dentistry procedures (tooth extraction, endodontic treatment, scaling and root planing, oral irrigation devices and others) infections of the oral cavity may act as a focus from which hematogenous spread of microorganisms may occur, and in presence of previous pathologies of the host, systemic infections might develop22.
We agree with Torres23, in using cultures on chromogenic media for the primary isolation of Candida species, because it allows the existence of other species to be observed more easily. Microorganisms have been isolated from teeth with pulp necrosis and clinically intact crowns. The teeth appear to be clinically healthy but reveal micro-fissures in hard tissues, which provide a means of entry for microorganisms. It has also been suggested that bacteria in the gingival crevice or periodontal pocket may reach the canals through periodontal blood vessels. Pulp infection may also occur through exposed dentin tubules at the cervical surface of the root due to lacunae in the cement covering. Microorganisms have also been found in necrotic pulp through blood circulation24. We agree with Nair24 that pulp necrosis might be associated under certain circumstances with fissures in the hard tissues or other means of entry. A thin, permeable epithelium (gingival fissure) enables microorganisms that are present to reach the connective tissue and blood capillaries. It should also be noted that the endoperiodontal habitat may undergo changes in pH, oxygen pressure, nutrient availability and a range of interactions of the microbiota that is present12. A review of the literature on candidiasis mentions the genus Candida and the species albicans as the most frequently identified, whereas the other species are found less frequently25. Our work agrees with that of Rodriguez Ortega25; in that C. albicans is the most prevalent species from oral mucosa samples, and in our study, a low number of a variety of other Candida species were identified, such as dubliniensis, guilliermondii, krusei, parapsilopsis, tropicales and glabrata. Sen et al.26 reported that C. albicans is the yeast that is most frequently isolated from samples from the dentin wall of root canals and was considered to be a dentinophillic microorganism. In our study, the most prevalent species from the periapical zone of canals with septic necrosis was Candida dubliniensis (10.98 %), followed by Candida albicans (6.1%).
The importance of identifying the genus and species in these endodontically originating processes is epidemiological surveillance, because they form reservoirs of opportunistic microorganisms, which, under certain clinical situations, take part in specific chronic diseases. The presence of Candida albicans has been found to be associated with the failure of root canal treatments, and irrigant solutions and intracanal medication are required to eliminate it27. An extensive review of the literature on endodontic microbiology showed that the different types of infections have poly-microbial etiology, and that they are usually represented by a mixed consortium whose diversity varies according to the type of infection. The profile of the microbial community shows great inter-individual variability, and the differences are even more pronounced when samples are taken from different geographic locations28.
Further studies should be undertaken on larger numbers of patients in order to analyze the role of Candida species in the biofilm of root canals and its relationship with different parts of the oral cavity in our environment.
CONCLUSION
1. Candida species were identified in single-root teeth with closed chronic periapical lesion with septic necrosis.
2. The species that was most frequently isolated from oral mucosa was Candida albicans, followed by Candida dubliniensis, while from the periapical zone of canals with septic necrosis it was Candida dubliniensis followed by Candida albicans.
ACKNOWLEDGMENTS
This work was supported by a Grant UBACYT O 016 from University of Buenos Aires.
1. Siqueira JF Jr. Endodontic infections: concepts, paradigms, and perspectives.Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;94:281-293. [ Links ]
2. Nair PN, Sj.gren U, Kahnberg KE, Krey G, Sundqvist G. Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 2000;16:580-588. [ Links ]
3. Nair PNR. On the causes of persistent apical periodontitis; a review. Int Endodont J 2006;39:249-281. [ Links ]
4. Donian RM. Biofilms: Microbial life on surfaces. Emerg Infect Dis 2002;8:881-889. [ Links ]
5. Peters LB, van Winkelhoff AJ, Buijs JF, Wesselink PR. Effects of instrumentation, irrigation and dressing with calcium hydroxide on infection in pulpless teeth with periapical bone lesions. Int Endod J 2002;35:13-21. [ Links ]
6. Horiba N, Maekawa Y, Abe Y, Ito M, Matsumoto T, Nakamura H. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg Oral Med Oral Pathol 1991;71:492-495. [ Links ]
7. Hashika K, Suzuki K, Yoshida T, Nekane A, Horiba N, Nakamura H. Relationship between clinical symptoms and enzyme-producing bacteria isolated from infected root canals. J Endod 1994;20:75-77. [ Links ]
8. Maita E, Horiuchi H. Polyamine analisis of infected root canal contents related to clinical symptoms. Endod Dent Traumatol 1990;6:213-217. [ Links ]
9. Harriott M, Noverr MC. Candida albicans and Staphylococcus aureus from polimycrobial biofilm:effects on antimicrobial resistance. Antimicrob Agents Chemother 2009;53:3914-3922. [ Links ]
10. Friedman S. Prognosis of inicial endodontic therapy. Endodontic Topics 2002;2:59-88. [ Links ]
11. Munson MA, Pitt-Ford T, Chong B, Weightman A, Wade WG. Molecular and cultural analysis of the microflora associated with endodontic infections. J Dent Res 2002;81:761-766. [ Links ]
12. Waltimo T, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontics yeast infection. Endodontic Topics. 2004;9:66-78. [ Links ]
13. Adam B, Baillie G, Douglas LJ. Mixed species biofilms of Candida albicans and Staphylococcus epidermidis. J Med Microbiol 2002;51:344-349. [ Links ]
14. Svensater G, Bergenholtz G. Biofilm in endodontic infections. Endodontic Topics. 2004;9:27-36. [ Links ]
15. Thomas N, Nakaes L. Managing the complexity of dynamic biofilms J Dent Assoc 2006;137(suppl);10S-15S. [ Links ]
16. Brown PD. Surgery for infective endocarditis. Curr Infect Dis Rep 2007;9:291-296. [ Links ]
17. Sritharan M, Sritharan V. Indian Emerging problems in the management of infectious diseases: the biofilms. J Med Microbiol 2004;22:140-142. [ Links ]
18. Listgarten MA, Lai CH, Young V. Microbial composition and pattern of antibiotic resistance in subgingival microbial samples from patients with refractory periodontitis. J Periodontol. 1993;64:155-161. [ Links ]
19. Ortiz Moncada C. Interpretacion clinica de las lesiones endoperiodontales. Medisan 2002;16 (2) http://www.sld.cu/revistas/san/vol16 [ Links ]
20. Jewtuchowicz VM, Brusca MI, Mujica MT, Gliosca LA, Finquelievich JL, Iovannitti CA, Rosa AC. Subgingival distribution of yeast and their antifungal susceptibility in immunocompetent subjects with and without dental devices Acta Odontol. Latinoam 2007;20:7-22. [ Links ]
21. Nasia Safdar MD, Dennis G, Maki MD. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, Enterococcus, Gram-Negative Bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002;136:834-844. [ Links ]
22. Philip S. Stewart Mechanisms of antibiotic resistance in bacterial biofilms International. J Med Microbiol 2002; 292:107-113. [ Links ]
23. Torres Rodriguez JM. Micologia Medica. Spain: Editorial Masson, 1993 [ Links ]
24. Nair PNR. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med 2004;15:348-381. [ Links ]
25. Rodriguez Ortega J, Miranda Tarrago J. Candidiasis de la mucosa bucal. Revision bibliografica. Rev Cubana Estomatol 2002;39:1-18. [ Links ]
26. Sen BH, Safavi KE, Spanberg LS.Groowth pattern of Candida aalbicans in relation to radicular dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endodod 1997; 84:68-73. [ Links ]
27. Ashraf H, Samiei M, Eslami G, Ghodse Hosseini MR. Presence of Candida Albicans in root canal system of teeth requiring endodontic retreatment with and without periapical lesions. Iranian Endodontic Journal 2007;2:24-8. [ Links ]
28. Siqueira JF Jr, Rôças IN. Diversity of endodontic microbiota revisited. J Dent Res 2009;88:969-981. [ Links ]














