INTRODUCTION
Periodontal disease is an infectious disease characterized by accumulation of dental biofilm, inflammation of tooth supporting tissues, and formation of periodontal pockets. It can lead to alveolar bone resorption and loss of periodontal attachment tissue 1 . In most cases, periodontitis entails chronic progression, and under certain circumstances, advanced stages of the disease can cause tooth loss. Destruction of periodontal tissues is mainly due to complex interactions between pathogenic bacteria toxins and host-derived mediators generated during immuno-inflammatory response 2 , 3 . Exposure to bacterial products such as lipopolysaccharide (LPS) can trigger an inflammatory sequence of events 1 , 4 . LPS is known to stimulate the production of cytokines and other inflammatory mediators, and to promote the release of matrix metalloproteinases (MMP) from the host tissues, which are destructive for the extracellular matrix and alveolar bone 5 , 6 . In addition to degrading type I collagen (which is required for the access of osteoclasts to the resorption site) from the extracellular matrix, MMPs modify the biological activity of cytokines, chemokines and growth factors, leading to a proinflammatory pathway 7 , 8 . Nitric oxide (NO) is a free radical produced by the endothelium, which is involved in various physiological processes such as a protective role in the micro vasculature. However, excessive NO production is associated with tissue injury 9-10 . The ingress of inflammatory cells into sites of infection promotes the release of eicosanoids such as prostaglandin E2, which is known to be a potent stimulator of bone resorption associated with loss of periodontal attachment tissue 11 .LPS also stimulates host immune cells to produce interleukins, TNFa, acute-phase proteins, chemokines and adhesion molecules. These processes occur due to the interaction with toll-like receptor 4 (TLR4) expressed on the target cellmembranes 5 , via CD 14 protein, and to the activation of the nuclear factor kappa-B (NF-kB). These proinflammatory molecules are believed to induce several cellular reactions, which eventually lead to the inflammatory response of periodontal tissue and a consequent imbalance between alveolar bone formation and resorption, by favoring the latter. This resorption is mediated by receptor activator of nuclear factor-kappa B ligand (RANKL), its receptor RANK, and a decoy receptor osteoprotegerin (OPG) 12 , 13 . Furthermore, it is likely that bacteria contribute to the pathogenesis of periodontal disease by producing enzymes and other substances that could alter the tissues surrounding the periodontium and produce a toxic effect on their cells.
Saliva is a fluid constituted by 99% water, while the remaining 1% is formed by electrolytes, mucins, enzymes, growth factors and other organic molecules. All these components give saliva a vital role in the oral cavity, enabling initial digestion, swallowing, modulation of taste, speech, involvement in immunity, wetting and diluting substances, and maintenance of mucosal and hard tissue integrity 14-16 . Consequently, hyposalivation compromises the integrity of hard and soft oral tissues, enhancing new bacterial and fungal infections and the occurrence and progression of dental caries 17 , as well as causing ill-fitting dentures and taste alterations. It canalso alter speech, eating and swallowing, with serious harm to life quality 18 . Furthermore, the submandibular gland is involved in immune and inflammatory responses in both local and systemic disorders 19 .
Xerostomia is a sensation of dryness in the oral cavity produced mainly by insufficient saliva secretion from salivary glands. It affects millions of patients around the world, ranging from 1% to 30% of the population, or perhaps more, depending on the features of the population studied 20-21 . Xerostomia prevails in individuals over65 years of age. Its causes may be local, such assialadenitis, sialolithiasis, infections or neoplasm of the oral cavity and damage to the salivary gland tissue caused by radiotherapy; or systemic, including chronic autoimmune diseases such as Sjogren syndrome 22 , psychiatric or neurological disorders 23 , diabetes 24 , HIV 25 and drug or medicine intake 26 , among others. Studies on rat pathophysiology have implemented some experimental models of xerostomia, such as X-ray-induced xerostomia 27 . However, those models have seldom been studied in combination with experimental periodontitis 28 , and have never been used with LPS-induced periodontitis. Since submandibular glands (SMG) and sublingual (SLG) glands combined contribute to whole saliva volume by approximately 65% of stimulated saliva and almost 50% of resting to whole saliva volume 29 , submandibular-sublingual complexablationprovides a useful model to assess the impact of reduced salivary flow on oral structures. We hypothesize that periodontal disease or hyposalivation individually produce deleterious effects on periodontal tissues and mandibular bone, and that coexistence of both exacerbates the deterioration. The aim of this study was to compare the deleterious effects caused by hyposalivation, periodontitis, and the combination of both on mandibular bone and periodontal tissues in rats subjected to such experimental conditions.
MATERIALS AND METHOD
Animals
Three-month-old adult male Wistar rats (300-350 g) from the authors’ own laboratory colony were kept in group cages in an animal room with a 12-hour light photoperiod (0700-1900), room temperature maintained at 22°C-25°C, and free access to rat chow and tap water. The experimental procedures performed were approved by the Animal Care Committee of the Dental School of the University of Buenos Aires, Argentina (CICUAL-ODON/ FOUBA N° 013/2016), and conducted according to the European Communities Council Directive of 24 November 1986 (86/609/EEC). All animal experiments complied with the ARRIVE guidelines.
Design
Twenty-eight rats were distributed into the following four groups containing seven rats per group: 1) control rats, 2) rats subjected to experimental periodontitis (EP), 3) rats subjected to hyposalivation (H), and 4) rats subjected to hyposalivation and experimental periodontitis (H+EP). On day 0, groups Hand H+EP underwent bilateral submandibulectomy (SMx) under anesthesia consisting of intraperitoneal injection of ketamine hydrochloride (Holliday-Scott SA, 70 mg/kg body weight) and 2% xylazine hydrochloride (Konig Laboratories SA, 10 mg/ kg body weight), based on a previously described method 28 . A midline incision of 15 mm was made through the skin and fascia of the ventral surface of the neck, the excretory ducts and main blood vessels were tied off, and submandibular-sublingual salivary complexes were dissected free from their supporting connective tissue and removed. They were excised with preservation of the surrounding neural structures, including the marginal mandibular branch of the facial nerve and the hypoglossal and facial nerves.
In groups EP and H+EP, on day 7, periodontitis was induced by injecting 20 pl of LPS (1 mg/ml) from Escherichia coli (serotype 055-B5, Sigma-Aldrich) into the vestibular and lingual gingiva of the right and left mandibular first molars and into the interdental space between the first and the second mandibular molars (60 pl of LPS per tooth and120pl per rat each time of treatment) under inhalational sevoflurane anesthesia, while control and H rats received no injections during the experiments. This protocol of injections was executed for a period of 6 weeks on days 1, 3, and 5 of each week, based on a previously described method 30 . Gingival injections were placed with a 13-mm 27-gauge microfine insulin syringe. All the experiments were performed at least twice. The graphs represent the results obtained from one of them.
Micro-computed tomography (μCT) assessment
After a 48 hour-period of fixation in 4% paraformaldehyde solution and 10% 0.1M phosphate buffer (pH 7.4), hemimandible samples were scanned using the Skyscan 1172 (Bruker microCT) at 90 kV, 111pA, with a 0.5 aluminum + 0.038 copper filter and 36% beam hardening correction, ring artifacts reduction 13, 180 degrees of rotation and exposure range of 0.4 degree. Images were captured with 1304x1024 pixels and a resolution of 13.2pm pixel size. Projection images were reconstructed using the NRecon software (BrukerN Recon 1.7.1.0) and three-dimensional images were obtained by the CT-Vox software. Morphological parameters of trabecular bone microarchitecture were assessed employing the CTAn software following the guidelines recommended by Bouxsein et al. 31 .
One region of interest was confined to the first molar-interradicular trabecular bone and limited by its four roots. Tomograms were oriented in the axial direction, considering the most coronal part of the interradicular bone and the last tomogram which showed the four first molar roots, as the upper and lower borderlines, respectively. The working area was drawn among these four roots and redefined as tomograms progress. Measurements of axial images included bone volume fraction (bone volume/total volume, BV/TV, %), total bone porosity (Po-tot, %), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp., mm), trabecular number (Tb.N, 1/mm) and height of the periodontal space. Interradicular bone was also assessed through tomograms oriented in the parasagittal direction. Images for this evaluation were selected so that whole mesial and distal roots and bone located between them appeared in tomograms, while lingual and buccal roots did not. In the parasagittal images, the height of periodontal space was evaluated by finding the average of equidistant lines between bone surface and dental surface (BS-DS) in millimeters. Buccal and lingual cortical plates were studied by means of coronal images. Distances were measured between the end of the mesial root and the beginning of the distal root, as the anterior and posterior references. This was used as an approach to the first lower molar-cortical bone. Distance between the cement-enamel junction and alveolar crest (CEJ-AC) was measured, eliciting separately measurements from buccal and lingual faces of the first lower molar in ten tomograms per side. Results were expressed in millimeters.
Histomorphometric analysis
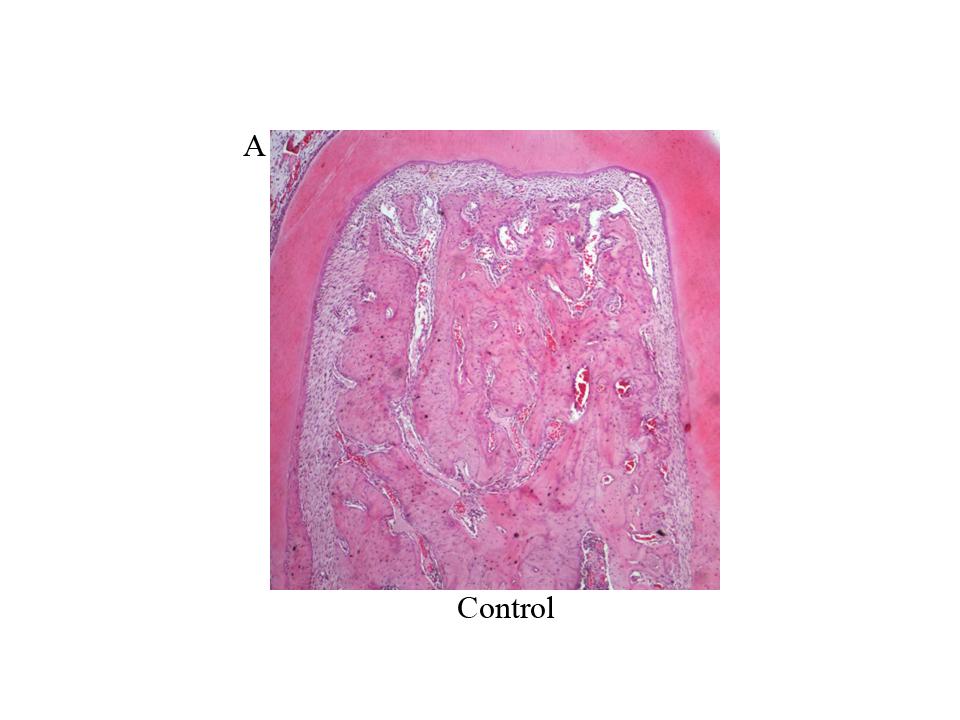
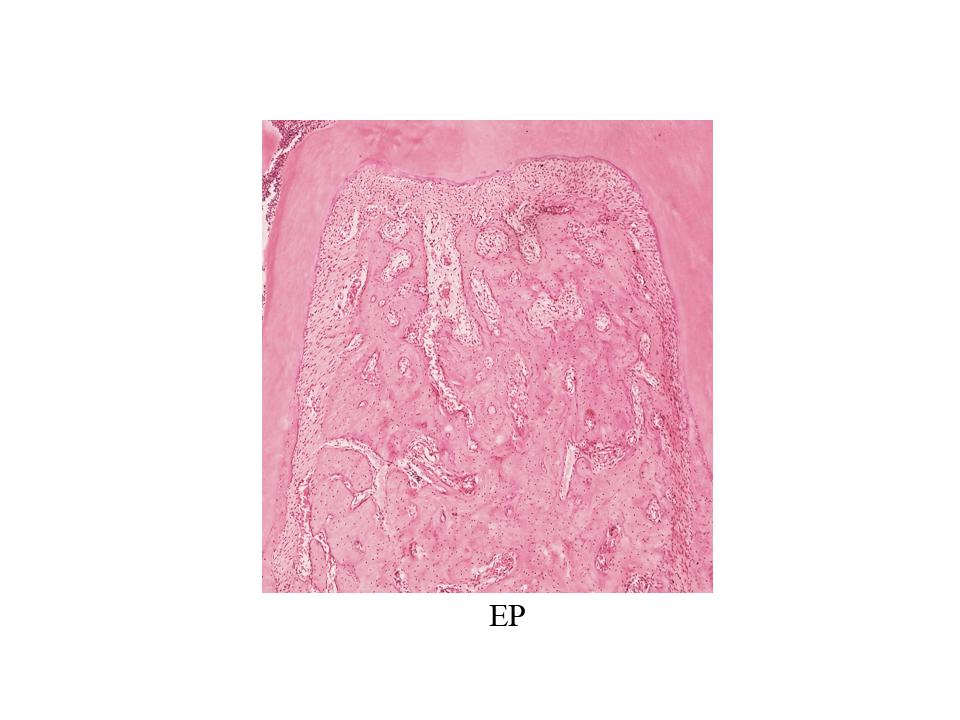
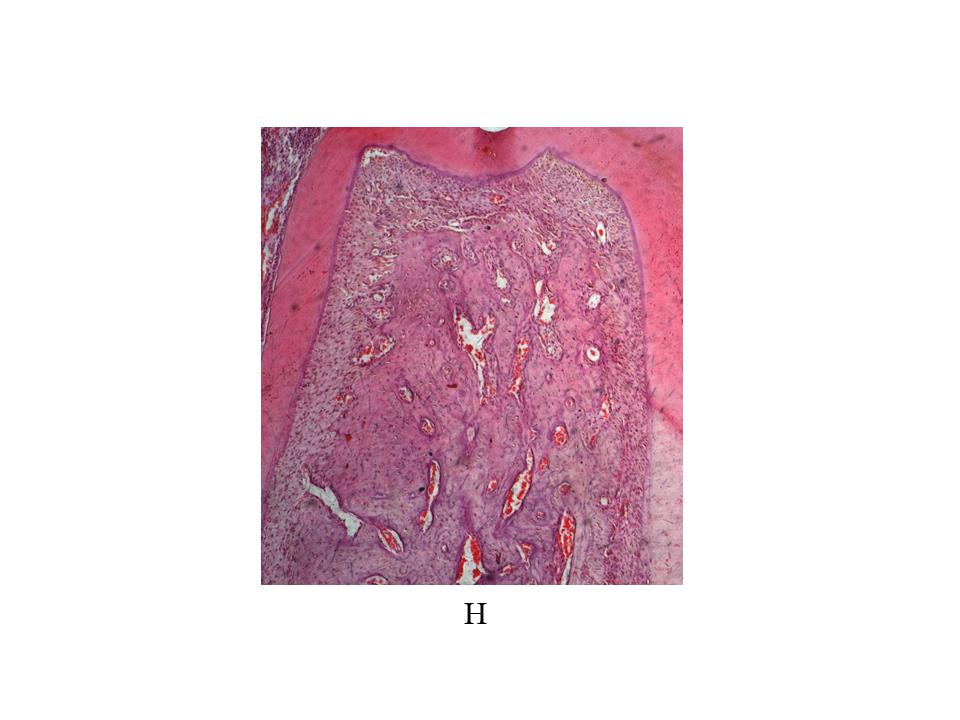
Hemimandibles were extracted and fixed in formalin buffer. After 3 days, they were decalcified in 10% EDTA pH 7 for 45 days, then dehydrated with ethyl alcohol and clarified with xylene. The sector containing the first molar of each decalcified hemimandible was embedded in paraffin at 56°C to 58°C. Sections oriented mesial-distally of each mandibular first molar were cut from the paraffin blocks under astereomicroscope with a Jung microtome (Leica Biosystems). Sections 5 gm thick were stained with hematoxylin and eosin (H&E), and a histomorphometric evaluation was performed on digitized microphotographs using imaging software (University of Texas Health Science Center). Bone histomorphometric analysis was conducted by determining bone formation and bone resorption surfaces on the coronal half of the interradicular bone.
Measurements of inducible nitric oxide (iNOS) activity
The activity of iNOS was measured in gingival tissue (GT) and SMG by modifying the method described by BredtandSnyder 32 , as follows: GTs were homogenized in 500 mLof cold20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonicacid (HEPES, Sigma-Aldrich, pH 7.4), with ethylene glycol-bis(2-aminoethylether)-N,N,N9,N9-tetraacetic acid (Sigma-Aldrich, 2 mM) and DL-dithiothreitol (DTT, Sigma-Aldrich, 1mM). A similar procedure was used for the SMG from control and EP groups, but it was homogenized in 600 mL of HEPES. After tissue homogenates were achieved, nicotinamide adenine dinucleotide phosphate (Sigma-Aldrich, 120 mM) and 200,000 dpm of [14C]-arginine monochloride (PerkinElmer,297 mCi/mmol) were added to each tube and incubated for 10 minutes at 37°C in a Dubnoff metabolic shaker (Thermo Fisher Scientific 50 cycles per minute; 95% O2/5% CO2) at 37°C. The tubes were then centrifuged at 10,000 g for 10 minutes at 4°C. Supernatants were applied to individual 1-mL resin columns (Dowex AG 50W-X8 Na+ form mesh 200400, Bio-Rad Laboratories) and washed with 2.5 mL of double-distilled water. The collected effluent fluid from each column was counted as activity of [14C]-citrulline in a Tri-Carb 2800TR Liquid Scintillation Analyzer (PerkinElmer). Since NOS converts arginine into equimolar quantities of NO and citrulline, data were expressed as picomoles of NO produced per minute per milligram of protein.
Determination of TNF-α
After extraction, gingival tissue was immediately homogenized in phosphate buffered saline containing protease inhibitor cocktail (Sigma-Aldrich) for mammalian tissue extracts to obtain the TNF-α preservation. Concentration of TNF-αwas determined using a sandwich enzyme-linked immunosorbent assay according to the manufacturer’s instructions. Briefly, in a 96-well ELISA plate, capture antibody, standards and samples, detection antibody, streptavidin-peroxidase (SAv-HRP) (enzyme) and tetramethylbenzidine (TMB, enzyme substrate),purchased from BD Pharmingen, were added sequentially. Data were expressed as picograms TNF-α per milliliter.
Radioimmunoassay of PGE 2
To determinePGE2content, gingival tissue was homogenized in 500 ml of absolute ethanol, and after centrifugation, supernatants were dried in a centrifugal vacuum concentrator (Speed Vac, Thermo Fisher Scientific) at room temperature. Residues were resuspended in buffer; and antiserum (sigma-Aldrich) was used as described in Mohn et al. (2011) 33 . Sensitivity of the assay was 12.5pg per tube. Cross-reactivity of PGE2 and PGE1 was 100%, but cross-reactivity of other prostaglandins was 0.1%. The intra- and inter-assay coefficients of variation for PGE2 were 8.2% and 12.0%, respectively. Results were expressed in picograms of PGE per milligram of wet weight.
Metalloproteinases by gelatin zymography
MMP-2 and MMP-9 enzymatic activity from gum were assessed using gelatin zymography. Briefly, a homogenate of gingival tissue from unilateral upper and lower first molar was obtained and placed in a 7.5% polyacrylamide gel containing0.1% sodium dodecyl sulfate (SDS) and 1 mg/ml purified gelatin (G8150, Sigma-Aldrich), as a MMP substrate. This gel was submerged in an electrophoretic cell along with a running buffer. After this step, gels were washed and then included in an appropriate buffer for 24 hours at 37°C to allow proteases to degrade the gelatin. Gelatin was stained with Coomassie blue solutionR-250 (Imperial Chemical Industries plc) in order to recognize the clear bands related to enzymatic activity, and subsequently subjected to a bleaching solution. MMP-2 and MMP-9 were identified through the corresponding molecular weight of proteolytic bands, by comparison with commercial standards (Rainbow Marker, Amersham ECL). Stain band intensity was determined by densitometry, using Image J software, and results were expressed in arbitrary densitometry units. Protein concentration was determined by the Bradford method 34 . Salivary gland homogenates were used as positive controls because they have a high concentration of MMP-2 and MMP-9.
Salivary secretion assessment
Rats were removed from cages 1 week before euthanasia and anesthetized with an intraperitoneal injection of ketamine hydrochloride (Holliday-Scott SA, 70 mg/kg body weight) and 2% xylazine hydrochloride (Konig Laboratories, 10 mg/kg body weight). Then, a dose of pilocarpine (Sigma-Aldrich, 0.5 mg/kg body weight) was administered intraperitoneally to induce salivation, and a cotton ball was immediately placed under the tongue of the rat to take up the total salivary secretion. This was determined as the difference in weight of the cotton ball before and after collection. Saliva was collected for 90 minutes after the administration of pilocarpine.
Biomechanical assessment
The mandibles of each rat were cleaned of adherent soft tissue and stored at -20°C wrapped in gauze soaked in Ringer’s solution. Mechanical properties of right hemimandibles were determined by a three-point bending mechanical test on an Instron Universal Testing Machine Model 4442 (Instron Corporation) to obtain the load/deformation (W/d) curves. The head used to load the bone was a rectangle of 12 mm x 2 mm. Each bone was placed on two lower supports, with the lateral side facing down and centered along its length. The supports were equidistant from the bone ends and separated by a constant distance L (distance between supports = 11 mm), equivalent to not more than two fifths of the bone length. Loads were applied transversally to the bone axis at a point immediately posterior to the posterior surface of the third molar at a rate of 5.00 mm/min, which is useful to describe the static properties of the bone structure. Responses to the loading obtained in the curve enabled analysis of the structural mechanical properties of the bones as a whole 35 , which are described below.
Load at the yielding point or elastic limit (Wy): represents the end point of the elastic deformation of the bone (yielding point) and defines a threshold about which unrecoverable permanent deformation occurs.
Structural stifíness or bone rigidity (Wydy): represents the slope of the elastic phase of the W/d curve and is a measure of the resistance of bone to deformation.
Structural strength (Wf): represents the value of the load at fracture and expresses directly the resistance of the whole bone to fracture.
Masseter muscle and hemimandible weights
After euthanasia, the superficial head of the masseter muscle and the hemimandible were identified, cleaned and weighed using a precision balance (Pioneer Ohaus). Results were expressed as relative weights: masseter muscle/animal weight or hemimandible weight/animal weight (mg/g).
Statistical analysis
Data were expressed as the mean of 6 determinations ± SEM per group. Results were evaluated by Two-Way ANOVA followed by the Tukey’s multiple comparisons test for unequal replicates. The sample size was set to enable the treatment differences with a similar magnitude to the experimental error with 80% power in the ANOVA test. All analyses were conducted with Prism software (GraphPad Software). Differences with P valúes <0.05 were considered statistically significant. Columns with distinct letters indicate a significant difference between groups.
RESULTS
Micro-computed tomography (μCT) assessment
In the interradicular analysis of the alveolar bone, groups H, EP and H+EP had lower valúes of bone volume fraction (BV/TV) and higher values of periodontal space height and total porosity (Po-Tot) than the control group. In turn, H+EP had lower bone volume fraction and higher total porosity value than group H, while these parameters did not differ significantly between groups H and EP ( Table 1 ). The height of the periodontal space in the interradicular area increased in groups subjected to H, EP and H+EP, with respect to Controls, but differences were not significant ( Fig. 1A ). Groups H and EP had a reduced trabecular number (Tb.N) compared to controls, although this reduction was not statistically significant when H+EP was compared with the control group. Trabecular thickness (Tb. Th) and trabecular separation (Tb.Sp.) did not differ among the experimental groups, with the exception of the lower trabecular thickness in H+EP than in the other three groups ( Table 1 ).




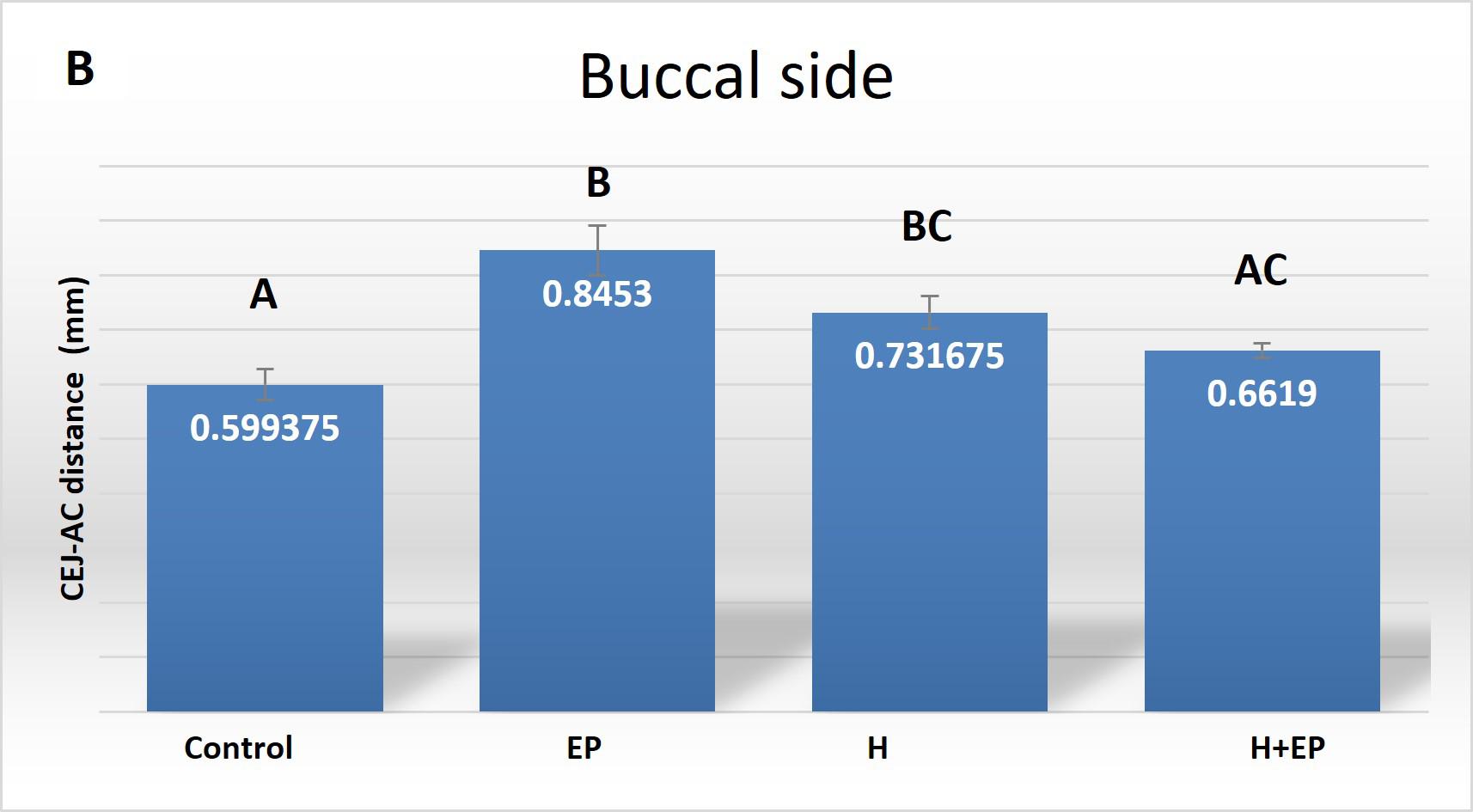
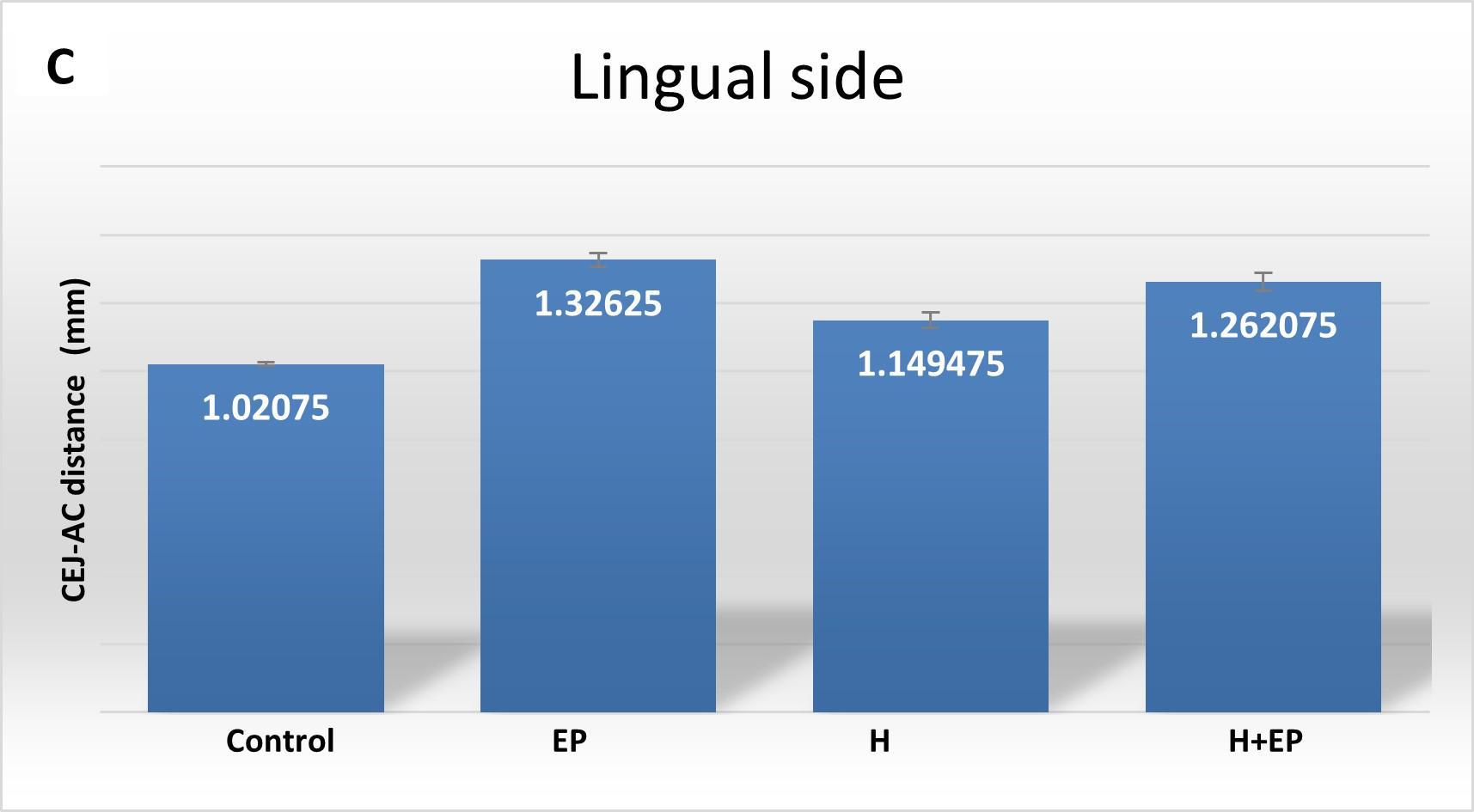
Fig. 1 A) Mesiodistal microtomographic images of the first lower molar, with specialfocus on the interradicular bone. Micro-com-puted tomography (ftCT) evaluation of alveolar bone loss in cortical plates: B) buccal side and C) lingual side. Results are pre-sented as mean ± SEM.
In the analysis of the cortical bone, rats subjected only to H or EP had longer CEJ-AC distance than controls on both lingual and buccal sides, which indicates higher alveolar bone loss. The same detrimental effect was observed in the H+EP group on the lingual side, but not on the buccal side. Group H had greater distance than control on both the buccal and the lingual side, but it was lower than in groups EP and H+EP on the lingual side ( Fig. 1B-C ).
Histomorphometric analysis
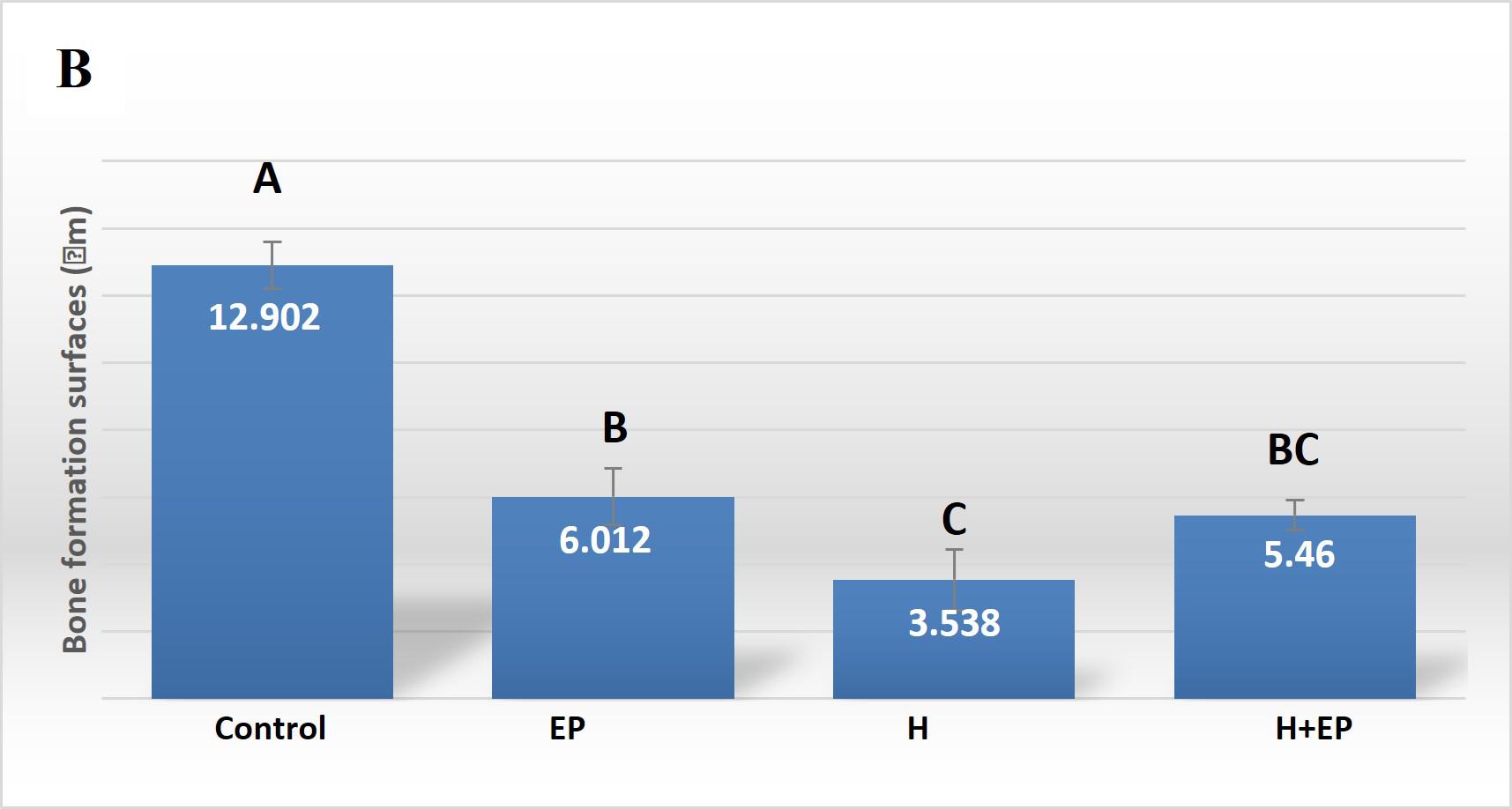
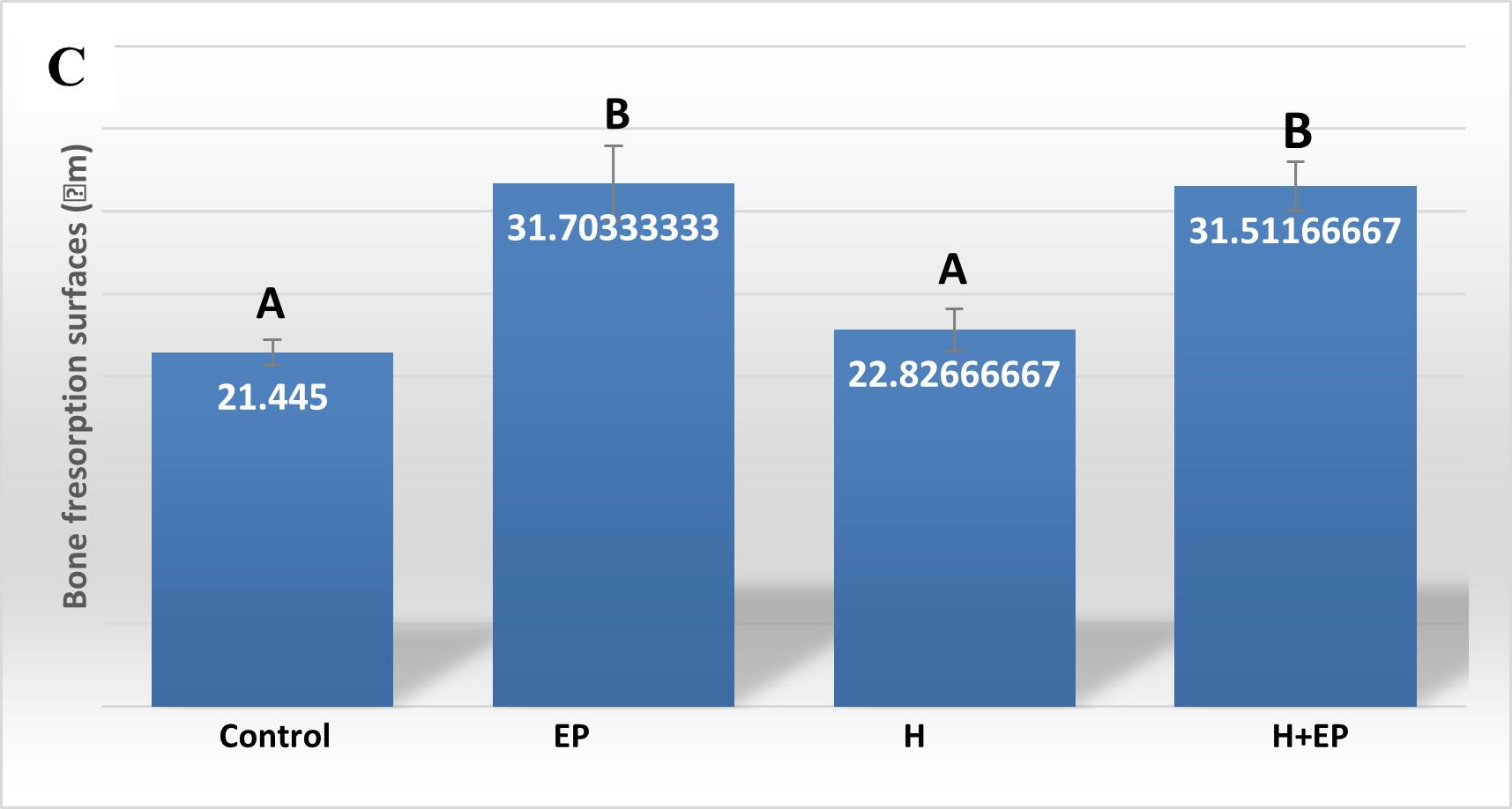
Histomorphometric analysis of the interradicular area, showed smaller formation surfaces in groups H, EP and H+EP than in the control. No statistical differences were found among the three experimental groups. Bone resorption surface values were higher in groups EP and H+EP than in group H and control. Mean resorption surfaces were very similar in control and H, as well as in EP and H+EP ( Fig. 2A-C ).
Measurements of inducible nitric oxide (iNOS) activity
iNOS activity in the gingival tissue increased in groups H, EP and H+EP with respect to control. It was higher in group H+EP than in groups H and EP ( Fig. 3A ).
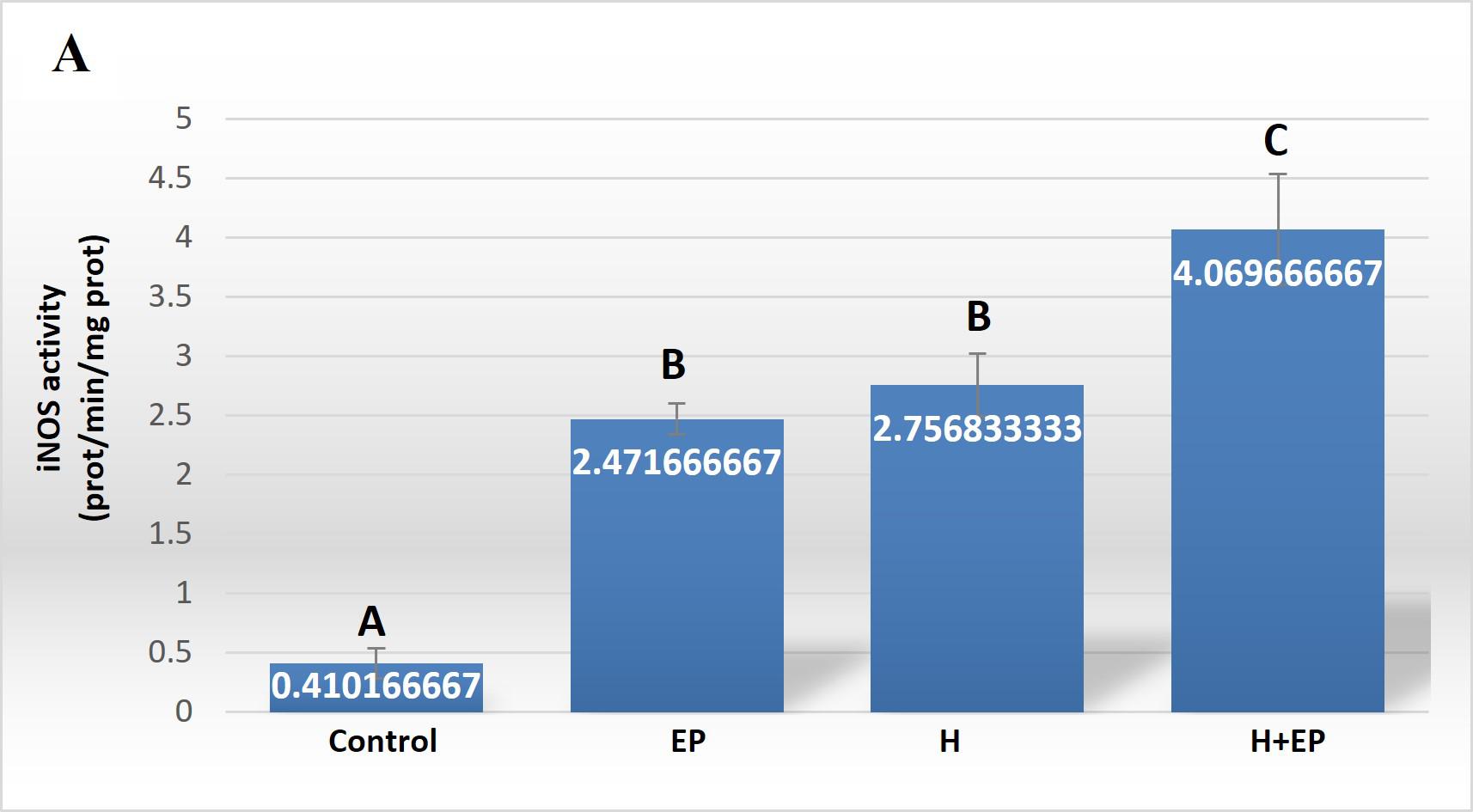
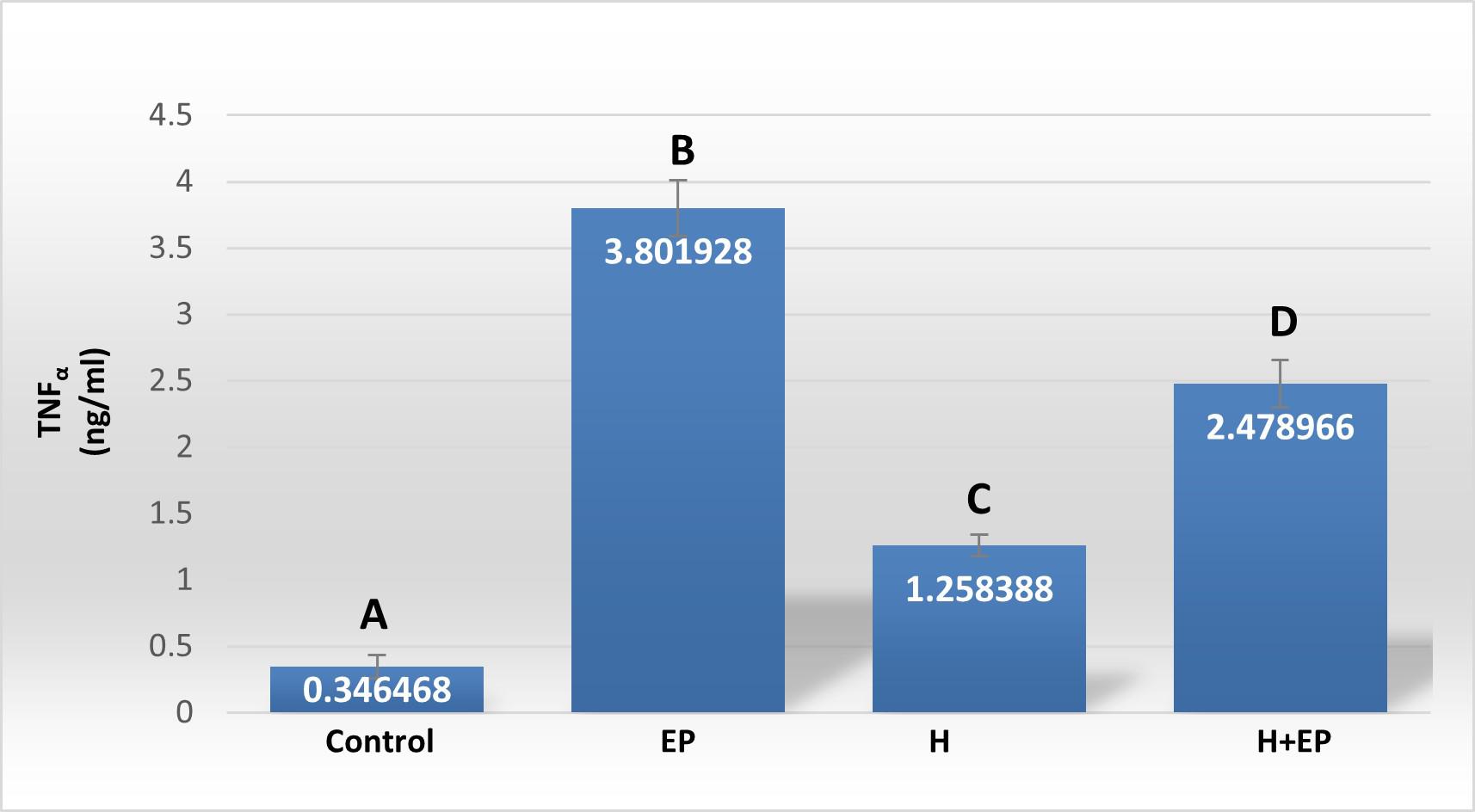
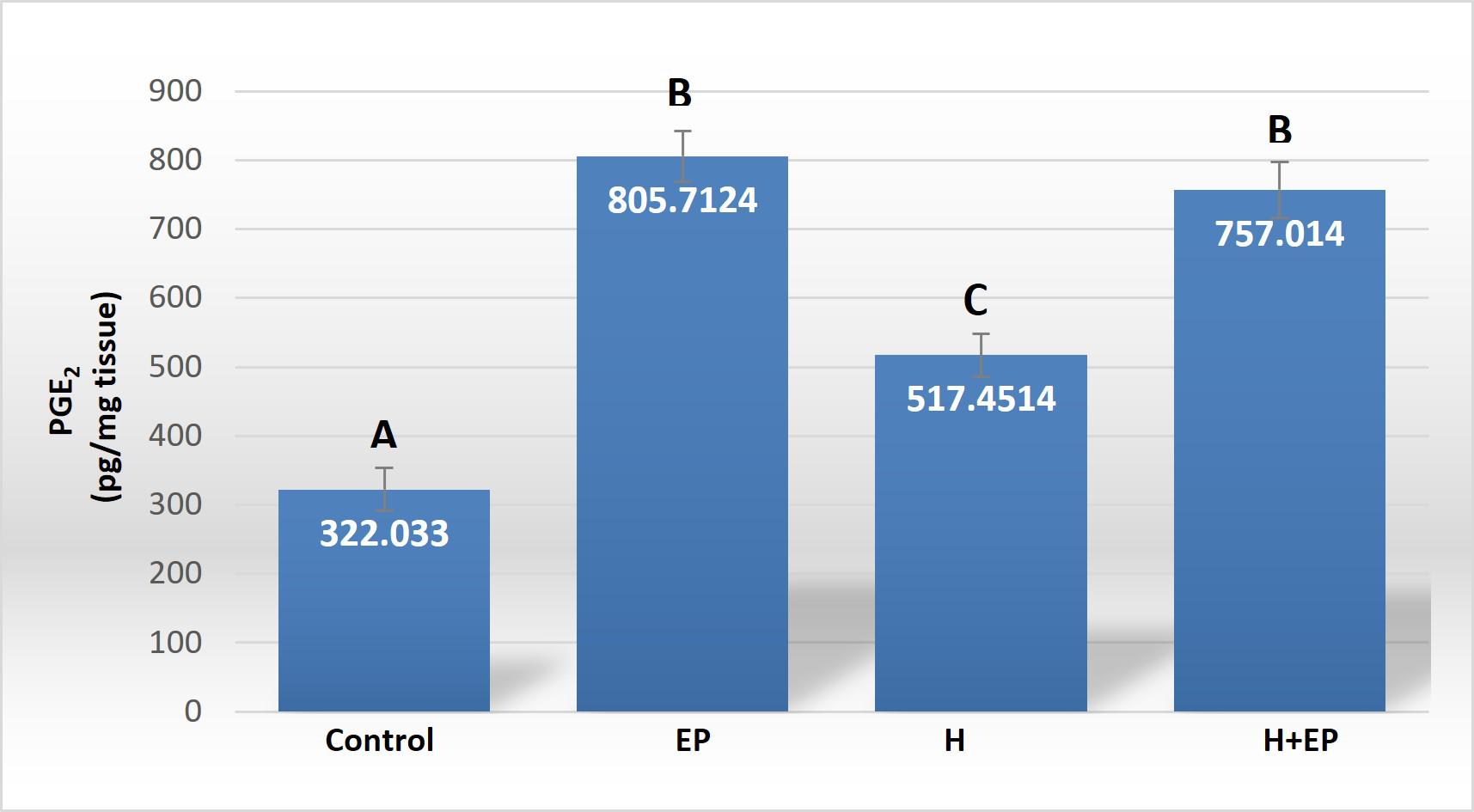
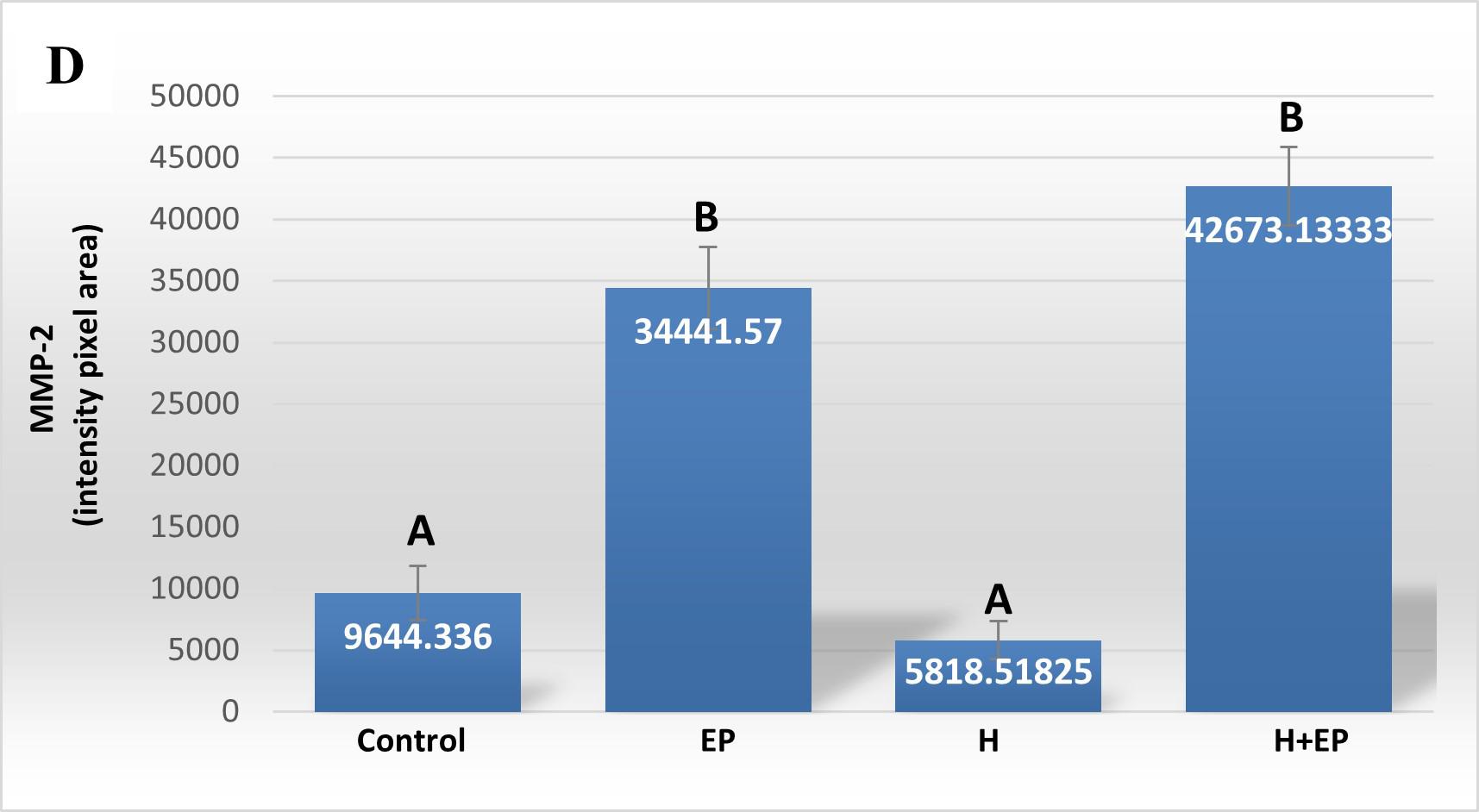
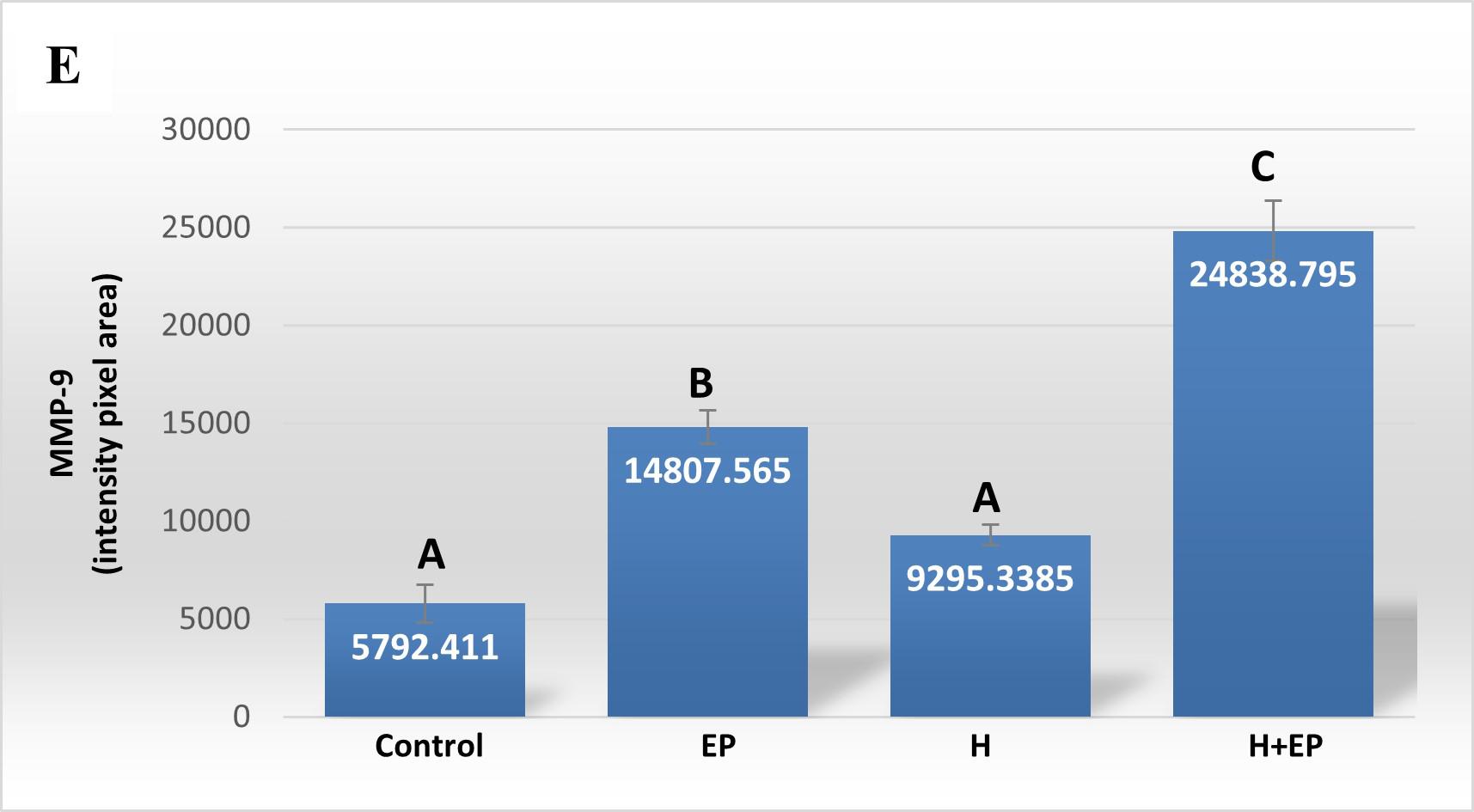
Fig. 3 Inflammatory mediators in gingival tissue: A) iNOS activity, B) TNFα level, C) PGE2 content, D) extracellular matrix metalloproteinase 2 activity (MMP2) and E) extracellular matrix metalloproteinase 9 (MMP9) activity. The unit of measurement is the intensity of the pixel area. Results are presented as mean ± SEM.
Determination of TNF-α
TNF-α level in gingival tissue was much higher in groups EP and H+EP than in the control group. Group H showed a lower level of TNF-α than the other two experimental groups, but it was significantly higher than in the control group ( Fig. 3B ).
Radioimmunoassay of PGE 2
Like other inflammatory mediators, gingival PGE2content level was higher in groups H, EP and H+EP than in controls, with no significant differences between groups H and H+EP ( Fig. 3C ).
Metalloproteinases by gelatin zymography
Extracellular matrix metalloproteinases 2 and 9 (MMP2 and MMP9) were measured in gingival tissue next to the first molar. Both groups with periodontitis, EP and H+EP, showed greater activity of MMP2 and MMP9 with respect to control and H groups. No differences were found between control and H groups. The gingival tissue of group H+EP showed higher MMP9 activity than group EP, but not higher MMP2 activity ( Fig. 3D-E ).
Table 1 Micro CT evaluation of interradicular bone
| Group | BV/TV % | Tb.Th mm | Tb.N 1/mm | Tb.Sp mm | Po(tot) % | Height of the periodontal space |
|---|---|---|---|---|---|---|
| Control | 74.5±2.1 A | 0.15±0.01 A | 4.9±0.3 A | 0.08±0.01 | 26.0 ±2.2 A | 0.144±0.02 A |
| EP | 65.2±2.6 BC | 0.16±0.02 A | 4.1±0.5 B | 0.10 ±0.01 | 34.8±2.7 BC | 0.260±0.01 B |
| H | 69.0±2.8 B | 0.17±0.02 A | 3.9±0.2 B | 0.09±0.00 | 31.0±2.9 B | 0.221±0.02 B |
| H+EP | 61.5±1.7 C | 0.12±0.01 B | 4.8±0.2 A | 0.09±0.00 | 38.5±1.8 C | 0.229±0.02 B |
Salivary secretion assessment
Total collected saliva was significantly reduced in animals subjected to experimental periodontitis as compared to controls, after 90 minutes of pilocarpine administration. As might be expected, the two groups subjected to submandibulectomy, H and H+EP, experienced a strong reduction of salivary secretion in comparison to controls, and even with respect to group EP ( Table 2 ).
Biomechanical analysis
Load at fracture, load at yielding and rigidity were higher in group H than in control, EP and H+EP. These biomechanical parameters did not differ significantly between groups EP and H+EP, or between them and the control group ( Fig. 4A-C ).
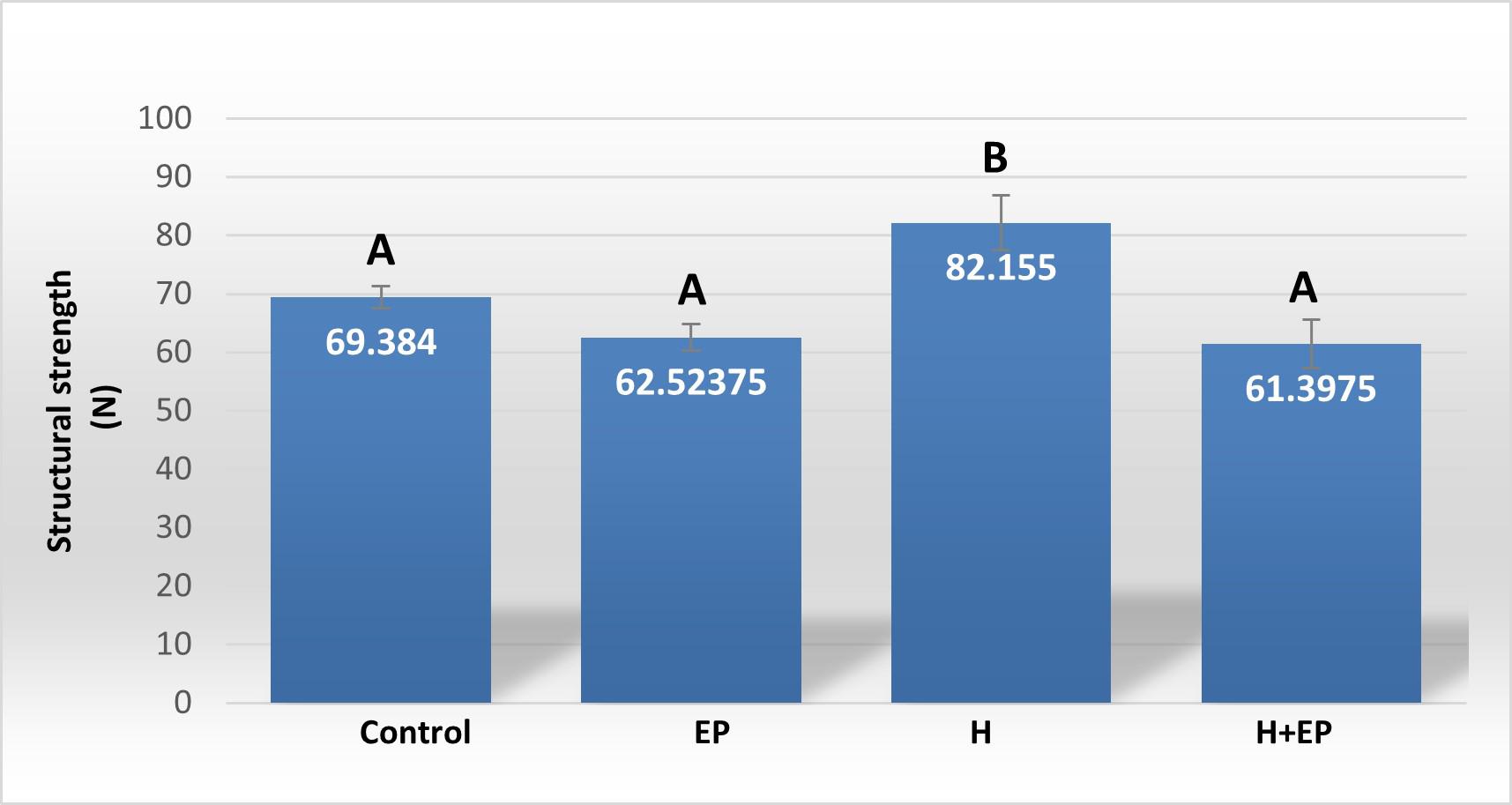
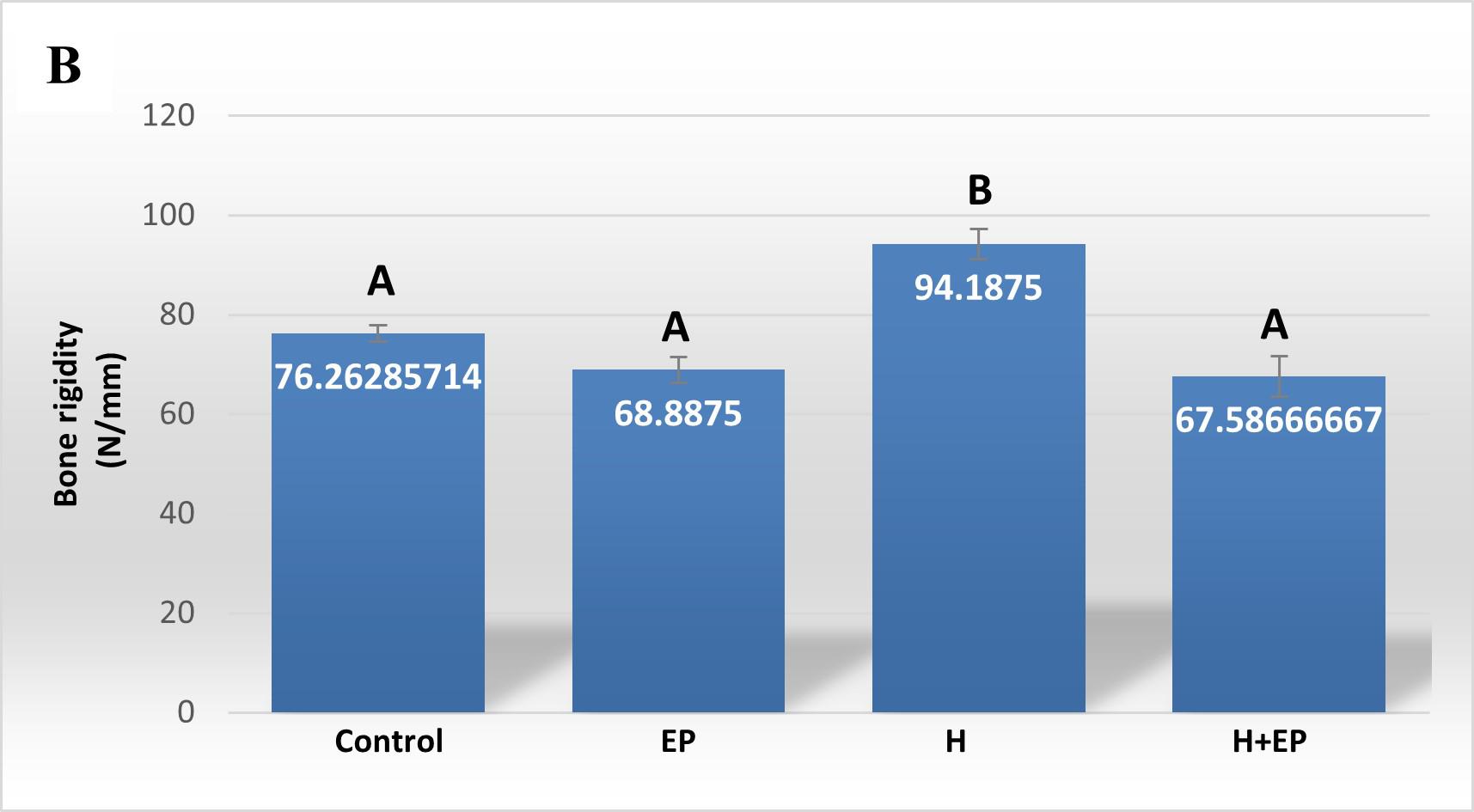
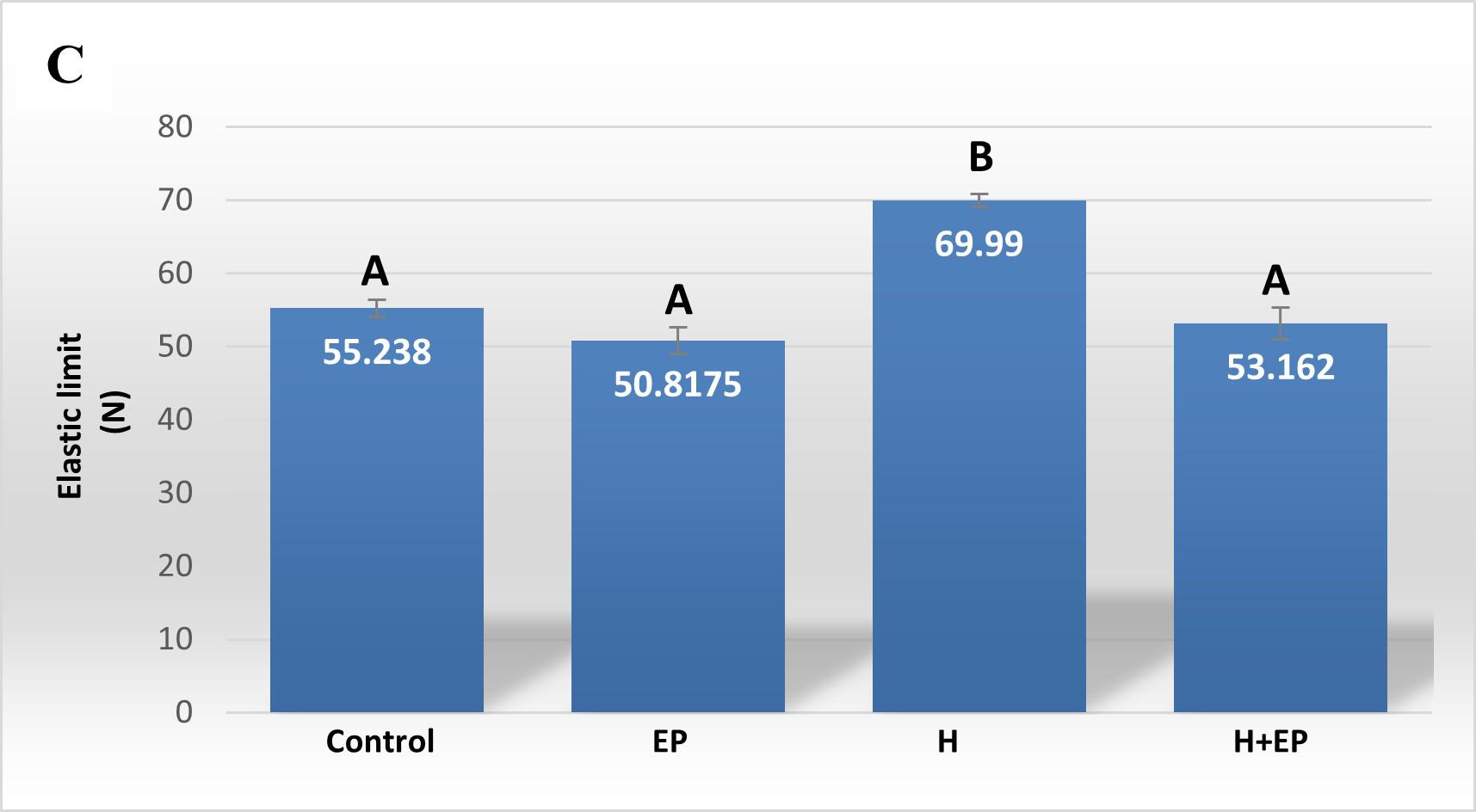
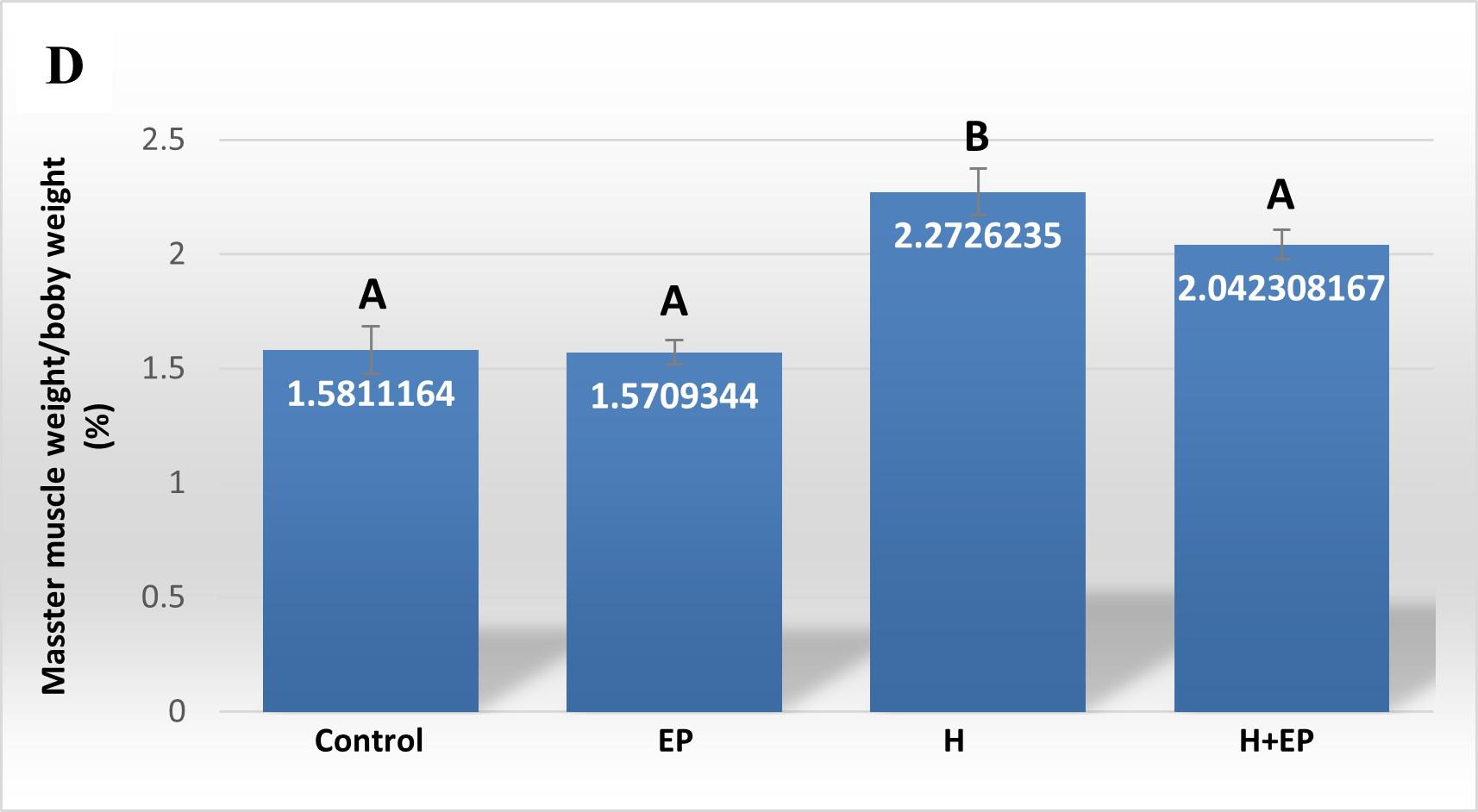
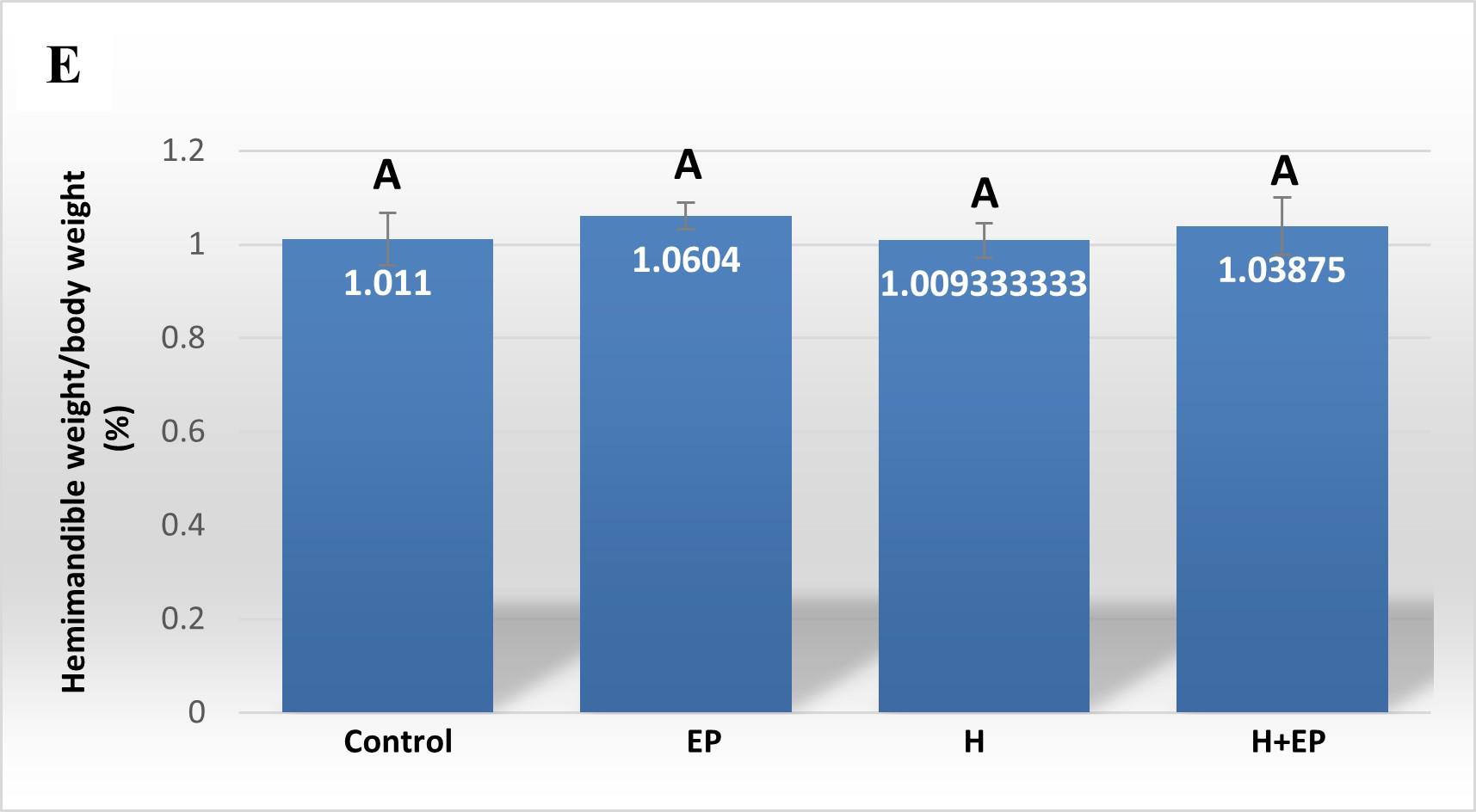
Fig. 4 Biomechanicalproperties of hemimandible. A) Structural strength or load atfracture; B) structural stiffness or bone rigidi-ty, and C) load at the yieldingpoint or elastic limit. Weights of masseter muscle (D) and hemimandible (E) as a percentage of body weight. Results are presented as mean ± SEM.
Masseter muscle and hemimandible weights
Superficial head of masseter muscle weight did not differ between control and EP groups. However, groups H and H+EP had greater masseter muscle weight than the two groups without submandibulectomy, which did not differ significantly from each other. None of the experimental groups presented variations in the weight of the hemimandible with respect to the control group (Figs. 4D and E).
DISCUSSION
Restorative effects of saliva have been widely demonstrated both in mucosa and dental tissues, though they are still currently under study 36 , 37 . In another study published by our laboratory, Mohn et al. demonstrated that wound repair after early post-tooth extraction is delayed in rats subjected to SMx-induced hyposalivation while the local inflammatory process is longer in those sockets 38 . The present study analyzed changes in oral tissues of rats subjected to the same kind of experimental hyposalivation and compared them to those produced by experimental LPS-induced periodontitis.
Micro-computed tomography assessment showed that animals in group H had higher periodontal space height and total porosity, but lower bone volume and trabecular number in the interradicular bone than controls. On the buccal and lingual cortical plates, also known as marginal bone, the distance from CEJ to AC was higher in group H than in control. These changes in the interradicular bone area and external cortical plates evidenced increased alveolar bone loss caused by SMx-induced hyposalivation. Similarly, the group of animals subjected to EP also showed an increase in the height of the periodontal space, total porosity and CEJ-AC distance on buccal and lingual cortical plates, and a decrease in bone volume and trabecular number with respect to control. However, no differences were found between groups H and EP, with the exception of the lingual side, where alveolar bone loss was higher in group EP. One interesting point is that in the animals subjected to hyposalivation and periodontitis concomitantly, bone damage caused by both insults individually did not exhibit a clear additive effect. Instead, values of alveolar bone loss in the interradicular area and external cortical plates did not differ greatly from those caused by the induction of hyposalivation and periodontitis separately, with the exception of trabecular thickness, which was lower, and total porosity, which was higher in group H+EP than in control, H and EP.
Our results partially agree with Vacas et al. (2008) regarding two parameters of interradicular bone loss, since in both studies bone volume fraction and height of periodontal space were higher in rats subjected to hyposalivation or to experimental periodontitis than in controls, although no additive effects were observed in a third group combining both experimental conditions. However, results in marginal bone differ between the studies, since Vacas et al. reported an increase of marginal alveolar bone loss on the lingual side, but not on the buccal side, in first molars of rats subjected to both hyposalivation and periodontitis compared to the individual experimental conditions 28 . Such differences could be explained by the different periodontitis models used in each study (ligature versus LPS-induced periodontitis). Additionally, Vacas et al. (2008) used contralateral side as a control of ligature-induced periodontitis, which they call a sham 28 . By contrast, in our study, control of LPS-induced periodontitis was represented by a different group of rats, exposed to the same conditions as the others, without either H or EP induction.
It should be underlined that in preliminary studies, we worked with two additional groups called sham and sham+EP, where sham underwent simulated surgery in which the neck skin was cut as in submandibulectomy, but the submandibular and sublingual gland remained intact after suturing. These preliminary studies showed that sham surgery did not produce any additional change to that produced by submandibulectomy alone on alveolar bone and inflammatory parameters (data not shown). Based on these results and considering the rational use of animals recommended by CICUAL, we decided not to proceed with further studies using sham groups.
It should also be considered that the expected additive deleterious effect of hyposalivation and periodontitis on alveolar bone loss, which is not reflected in the results obtained for H+EP group, may already exist in H and EP groups. This could be a consequence of the reciprocal induction of these two pathological conditions, as it has been reported that hyposalivation causes periodontitis-like bone loss, while periodontitis induces hyposalivation 6 , 39 . Furthermore, periodontitis-induced hyposalivation was also demonstrated in the present study. Therefore, the additive effect of hyposalivation and periodontitis would be masked in the H+EP group, since it does not differ significantly from the H and EP groups regarding most of the bone loss parameters assessed. In other words, hyposalivation and periodontitis may both be present in each group with these individual conditions, as a result of a direct or indirect induction. Another possibility is that the concomitance of the actions of LPS and the low volume of saliva that alters oral homeostasis evokes compensatory effects that prevent the harmful additive effects, as a protective response of the organism.
The histological examination of the interradicular bone showed smaller formation surfaces in groups H, EP and H+EP in comparison to control, with group H value being the lowest, while groups EP and H+EP had larger resorption surfaces than either group H or control. Images showing increased bone resorption in experimental groups included an increase in the number of osteoclasts (data not shown). These microscopic changes in the alveolar bone agree with previous studies by our group and others, which particularly reported alveolar bone histomorphometric damage originated by experimental periodontitis 40 , 41 . On another note, this finding related to the increased resorption surfaces and decreased formation surfaces in groups H, EP and H+EP is consistent with μCT results, in which the three experimental groups displayed changes with respect to the control. Conversely, some parameters evaluated by μCT, such as trabecular thickness and total porosity, showed and apparent additive effect when H and EP were induced concomitantly, while no additive effect was noticed in reference to the bone formation and resorption surfaces.
In order to analyze the mandible bone performance under hyposalivation and LPS-induced periodontitis conditions, we evaluated its biomechanical properties. Group H had higher load at fracture, load at yielding and rigidity than control, EP and H+EP rats, suggesting higher strength, lower elastic deformation and higher stiffness. The valúes did not differ significantly among control, EP and H+EP. The effects of hyposalivation on the biomechanical properties of the mandible recorded in the current study are similar to those reported by Mohn et al. (2018), who also demonstrated the increase of bone mass in the mandible after sumandibulectomy, working with a model of tooth extraction 42 . This might be related to the fact that the lower saliva production in group H causes dry mouth, making chewing difficult, which would lead to an increase in bone biomechanical properties, as a result of an adaptation to the greater requirement. This hypothesis could be supported by the augmented masseter muscle weight in rats subjected to submandibulectomy in the present study. Another possible hypothesis is that there could be mediation by signaling of transforming growth factor beta (TGF-P), a cytokine involved in the resorption phase and in bone organic matrix disorders 43 . Since TGF-P has been found as a constituent of saliva 44 , hyposalivation could imply reduced promotion of its pathway, leading to altered bone properties in the oral cavity 45 . Moreover, the absence of biomechanical changes in mandible bones of group EP in the current study had been also reported in a previous study by of our group 46 . In addition, biomechanical properties did not increase in the group of animals exposed to both conditions, H+EP, which exhibited similar values to those of group EP. This could be related to the imbalance of bone metabolism revealed by histological studies, since groups EP and H+EP showed larger bone resorption surfaces and smaller bone formation surfaces than the control, while group H showed bone resorption surfaces similar to those of the control and the smallest bone formation surfaces with respect to the other three groups. These results suggest that when hyposalivation and periodontitis coexist, the biomechanical effect of hyposalivation fades, perhaps as a compensatory phenomenon. Inflammatory mediators were evaluated in gingival tissues around first molars. Groups exposed to hyposalivation, periodontitis or both conditions together exhibited higher levels of inflammatory mediators such as iNOS, TNFa and PGE2, compared to control. Moreover, rats subjected to experimental periodontitis mostly showed increased inflammatory parameters with respect to group H, except in iNOS activity, which only surpassed group H levels when experimental periodontitis and hyposalivation developed together. These results suggest that, although hyposalivation causes an increase in gingival inflammatory mediators, they increase more markedly in response to LPS-induced periodontitis. However, the pro-inflammatory effect provoked by experimental periodontitis could be at least partially promoted under hyposalivation conditions. Hori et al. (2021) recently reported that xerostomia increases mucosal inflammation and bone resorption in a model of ligation-induced peri-implantitis, although xerostomia does not cause osteoclastogenesis or bone resorption around implants not subjected to ligature 47 . On the other hand, in the current study, MMP2 and MMP9 also had higher activity in groups EP and H+EP than control and H groups. MMPs belong to a protein family involved in the degradation of extracellular matrix compounds, associated with the progression of dental caries, apical lessons, periodontal diseases and orthodontic treatments 48 . MMP2 and MMP9 act on several forms of collagen and their activation can be initiated by bacterial species such as Treponema denticola and Porphyromonas gingivalis 49-50 . Tissue imbalance resulting from molecule alteration, through the increase of inflammatory mediators plus the increase in MMP production, can lead to a complex tissue response characterized ultimately by bone deterioration.
The limitations of this study might be related to the experimental models used. LPS-induced periodontitis is an appropriate model for studying the chronic deleterious effects induced by the immune response during the disease; however, the damages induced by bacterial toxins do not occur due to the lack of biofilm accumulation on the teeth. Submandibulectomy consisting of submandibular and sublingual ablation does not exactly reproduce the hyposalivation frequently observed in patients, in whom secretory activity is reduced in both submandibular and parotid glands. However, both models used in this study are supported by the scientific community, as demonstrated by the large number of published works based on them28, 30, 38, 40, 42, 46.
In summary, the present study demonstrated that SMx-induced hyposalivation increased gingival inflammatory parameters and caused damage to both alveolar bone and soft periodontal tissues. EP generated higher levels of inflammatory mediators than H, leading to greater damage to periodontal tissues. The concomitance of H and EP did not show a clear additive effect, in slight divergence from one of our hypotheses. Further studies could be conducted to elucidate mechanisms through which deleterious effects of induction of periodontitis and hyposalivation do not add up as might be expected, considering that when H and EP coexist, instead of producing an additive result, the effect of one of them seems to prevail. Finally, hyposalivation seemed to produce more extensive deleterious effects on the oral cavity than periodontitis, since biomechanical alterations were added to periodontal damage.