INTRODUCTION
Throughout history parasites have been con sidered as harmful agents and pathogens; how ever, it is currently known that they can regu late abundance and alter the structure of the host community and the food chains (Luque & Poulin, 2007; Bautista et al., 2013; Iannacone & Alvariño, 2013; Chero et al., 2016; Cardoso et al., 2017). Parasites are also excellent biological indi cators to investigate the ecology, migration and population structure of marine fishes (Silva et al., 2017).
Scombrids are one of the most popular and edible fish families in the world, and are con stituted by mackerel, tuna, and bonito (Paxton, 1998; Bárcenas et al., 2021; Yemmen & Gargouri, 2022). Trophic biology studies show that epipe lagic scombrids are widely known as opportunis tic and generalist predators, which means that their diet is made up of many organisms from different levels of the food chain (Paxton, 1998; Olson et al., 2016). Internationally, the species of the Scombridae family have mainly been re viewed for systematic, zoogeographical purposes and some parasitological investigations; how ever, few studies have focused on the interac tion between ecological factors and the parasite community of a specific host (Chero et al., 2016; Miranda-Delgado et al., 2019; Santos-Bustos et al., 2020, 2021).
Scomberomorus sierraJordan and Starks, 1895 (Scombridae) is a pelagic fish that is dis tributed from southern California (United State of America), including the Galapagos Islands (Aguirre-Villaseñor et al., 2006) to Paita, Peru (Collette, 1995; Santos-Bustos et al., 2020). This species presents a migratory behavior, which is due to its diet based on sardines and anchoveta (Collette & Nauen, 1983, Moreno et al., 2011), making it a species that sustains temporary fishery based on migration (Lucano et al., 2011; Bárcenas et al., 2021).
Some studies on the parasite community of S. sierra have been developed from a systematic, taxonomic and ecological perspective in Mexico (Santos-Bustos et al., 2020; Bárcenas et al., 2021; Morales-Serna et al., 2021). In this sense, the annual variation of the helminth community of 151 individuals of S. sierra in Mazatlán, Mexico has been analyzed, recording 13 helminth taxa, with the best represented group being digeneans (Barcenas et al., 2021). Similarly, interannual and spatial oscillations in metazoan parasite communities has been evaluated over a 10-year period in 674 S. sierra specimens in four loca tions on the Pacific coast of Mexico, identifying 24 parasitic metazoan taxa. In both studies, the parasite communities were characterized by a high richness of ectoparasite species, with monogeneans and some didymozoid species be ing numerically dominant (Santos-Bustos et al., 2020). In South America and Peru few studies have focused on the metazoan parasitic fauna of S. sierra. Therefore, from the first evaluations to date, only 11 species of parasites have been re corded over the last 43 years, nine of them from Peru, three copepods Acantholochus nudiuscu lus (Cressey and Cressey, 1980), Caligus omissus Cressey and Cressey, 1980, and Cybicola buccatus (Wilson, 1922), one tapeworms Adenocephalus pacificusNybelin, 1931, three monogeneans Pseudaxine sp., Mexicotyle mexicana (Meserve, 1938) Lebedev, 1984, and Thoracocotyle croceaMacCallum, 1913, and finally, two trematodes Anaporrhutum sp. and Didymozoon sp. (Cressey & Cressey, 1980; Luque et al., 2016).
Taking the above into account, the present study aimed to analyze the ecological parame ters of the parasitic community of S. sierra from Northern Peru.
MATERIAL AND METHODS
Hosts
A total of 58 specimens of S. sierra were ob tained from artisanal fishery between October 2019 and August 2022 from Puerto Pizarro, Tumbes, Peru (13° 30’ 07” S; 80° 23’ 33” W). The fish were preserved and transferred through a cold chain with ice in coolers to the laboratory for further evaluation. The morphometric data of total length (TL) (cm), weight (g) and sex of each fish were recorded. The dissection of each individual consisted of an intensive search for ectoparasites in the gills, eyes, skin, operculum, fins, and oral cavity.
Metazoan parasites
Metazoan parasites were removed, trans ferred and separated in petri dishes and finally washed with 0.9% saline solution (Suthar et al., 2022). All parasites followed standard mount ing and staining procedures for each taxon. The helminths were fixed in a mixture of absolute ethyl alcohol (85%), formaldehyde (10%), and acetic acid (5%) for one hour and then trans ferred to a 14.8 mL glass container with etha nol at 70%. For the preparation of permanent mounting sheets, the helminths were placed for one hour in acetocarmine, and then removed from the dye and washed in 70% ethyl alcohol to eliminate excess dye, followed by dehydration in a graded ethanol series, cleared in clove oil, and finally mounted in Canada balsam. Copepod crustaceans were rinsed in pure glycerin for a period of two weeks (Hayward & Rohde, 1999; Eiras et al., 2006; Almeida & Almeida, 2014; Thaenkham et al., 2022; Wood et al., 2023). The identification of monogeneans, trematodes, and copepods parasites was based on specialized pub lications (Gibson et al., 2002; Kohn et al., 2006, 2007; Gibbons, 2010; Cohen et al., 2013; Luque et al., 2016; Eiras et al., 2017; Mendoza-Garfi et al., 2017; Smit et al., 2019). Each of the species found was deposited in the collection of Parasitic Helminths and Related Invertebrates (HPIA) of the Natural History Museum of the Federico Villarreal National University (MHN-UNFV) under the codes: MUFV: ZOO-HPIA: 206-214.
Data processing and statistical analysis
The ecological parasite prevalence indices (P%), mean abundance (MA) and mean inten sity (MI) of infection were calculated for all hosts (Bush et al., 1997; Bautista et al., 2015). The type of strategy used was determined according to the P% obtained from each parasitic species found in S. sierra as follows: 1) core species, strategy for species with a P% greater than 60%; 2) second ary species, strategy for species with P% within the range of 40% to 60%; 3) satellite species for species with P% between 5 and 40%, and finally, 4) rare species with P% less than 5% (Suthar et al., 2022). The specific importance index (SII) calculated as the importance of each parasite species in the ecological assemblage was used in order to obtain an integrated infection index of both ecological descriptors: SII = P% + (MA x 100), where: SII= Specific importance index, P% = Prevalence, MA= Mean abundance of infec tion (Minaya et al., 2021a).
The dominance frequency of each parasitic species was determined as the number of times that a parasitic species is dominant in all the fish hosts examined and the relative dominance fre quency of each parasitic species was calculated as the number of individuals of a parasitic species divided by the total number of individuals of all taxa in the parasitic infracommunity. Single and multiple parasitic infection (two to three para sitic metazoan species) were determined (Rodhe et al., 1995).
For the parasite species presenting preva lences greater than 10% (Esch et al., 1990), dispersion indices were used (DI: Variance (S2) / MA, Poulin discrepancy index (PDI) and K of the negative binomial equation with its respec tive Chi square value (X2) to determine the type of distribution and degree of aggregation, which was classified as aggregate, uniform or random (Bego & Von Zuben, 2010). The calculations were made using the Quantitative statistical package. Parasitology 3.0 (Rózsa et al., 2000) The inter annual variation of the parasitic fauna was not evaluated due to the low number of specimens evaluated per year.
The Pearson’s correlation coefficient was applied to evaluate the association between TL versus P% and Weight versus P%, where the P% values were previously transformed to the square root of arcsine. The Spearman’s correla tion coefficient (rho) was used to determine the relationship between host TL and MA and MI and weight vs. host MA and MI of each parasite species, after calculating four ranges of TL (cm) (I: 13-49; II: 49.1-58; III: 58.1-67; IV: 67.1-78) and seven ranges of weight (g) (I: 301-398; II: 398.1-486; III: 486.1-574; IV: 574.1-662; V: 662.1-750; VI: 750.1-838; VII: 838.1-926). In all cases, the normality of the data was verified using the Kolmogorov-Smirnov test with the Lillierfors modification and the homocesticity of variances based on the Levene test (Stockemer, 2019; Minaya et al., 2020).
The Student’s t test was used to compare the MA and MI of each parasite and the sex of the host. 2x2 contingency tables were used to calcu late the degree of association between the sex of the host and P% of each parasite using the X2 and the Likelihood Ratio test. The analysis of the parasites in relation to the TL and the sex of the host was carried out only for species with a prevalence greater than 10% (Esch et al., 1990).
The following alpha diversity indices were de termined: Richness (S), Individuals, Menhinick (DMn), Margalef (DMg), Shannon-Weaver (H`), Pielou Equitability (J`), Simpson (D), Berger-Parker (d) and finally Chao-1 for the parasitic community component, for males and females (Salmerón-López et al., 2017; Minaya et al., 2021a; Negrelli et al., 2021). Similarly, to compare the values of H and D between sexes, the Student’s t test was used. The level of significance was evalu ated at a level of p≤0.05. To determine the diver sity indices, we used the statistical package PAST-Palaeontological STatistics, ver. 4.03, and for the descriptive and inferential statistics, the statis tical package IBM SPSS Statistics 27 was used.
RESULTS
The population structure of S. sierra was composed of 50% males (n=29) and 50% females (n=29). The TL of the 58 hosts ranged between 13-78 cm (mean ±standard deviation [SD] = 54.1 ± 9.2 cm). Males ranged from 40-78 cm (53.8 ± 8.2 cm) in length while females ranged between 13-76 cm (54.3 ± 10.3 cm).
A total of 58 individuals of S. sierra were examined, of which 1085 specimens of ectopara sites distributed in nine morpho-species were col lected and identified. The greatest richness was for the group of ectoparasitic trematodes which presented four species (Didymocylindrus sp., Glomeritrema sp., unidentified Didymozoidae, and Didymozoon sp.), followed by monoge neans, with three species [T. crocea, Mexicotyle mexicana, Scomberocotyle scomberomori (Cuvier, 1829)], and finally, the copepods with two spe cies (C. buccatus and Caligus pelamydis Krøyer, 1863) (Table 1). At least one species of metazoan parasite was found in 98% of the fish (n=57), while the remaining 2% (n=1) did not present any parasite. No endoparasites were recorded in S. sierra.
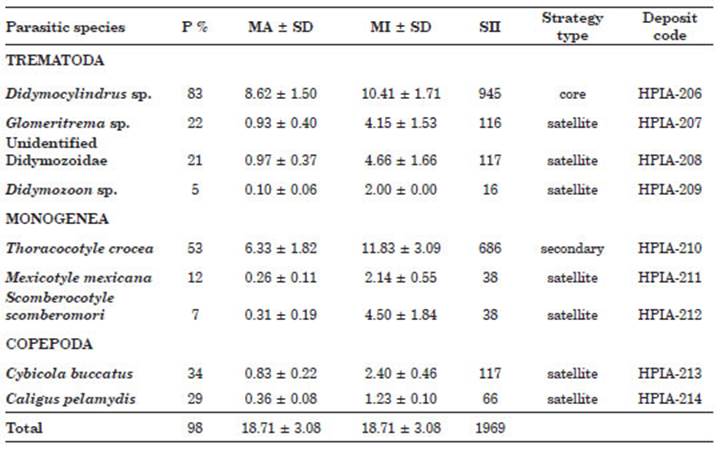
Table 1 Ecological descriptors of the gill ectoparasites found in the Pacific sierra Scomberomorus sierra from Puerto Pizarro, Tumbes, Peru. Abbreviations: P%, prevalence percentage; MA, mean in fection abundance; MI, mean infection intensity; SD, standard error; SII, specific importance index.
Site of infection, P%, MA, MI of infection, SII and type of strategy of the nine ectoparasitic metazoan species were shown for S. sierra (Table 1). The species with the highest P%, and MA was Didymocylindrus sp. followed by T. crocea with the highest MI (Table 1). Regarding the type of ecological strategy, one species was defined as a core species (11.11%), one as secondary (11.11%) and seven were cataloged as satellite species (77.77%) (Table 1).
The highest frequency of absolute domi nance for one species, for two or more species and the relative frequency was for the trematode Didymocylindrus sp., followed by the monogenean T. crocea. All other species showed a domi nance frequency of two or more species, except for M. mexicana, C. buccatus and C. pelamydis (Table 2).
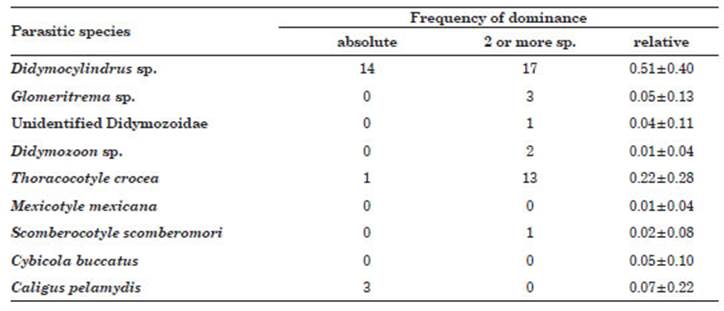
Table 2 Frequency of absolute dominance and relative dominance for one and two ectoparasitic spe cies in Scomberomorus sierra acquired in Puerto Pizarro, Tumbes, Peru.
The dispersion of seven ectoparasites of S. si erra based on three aggregation indices, DI, PDI and K, the latter with its interpretation by X2, for species with P% above 10% is shown. The three indices suggest high levels of aggregation for the parasites (Table 3).
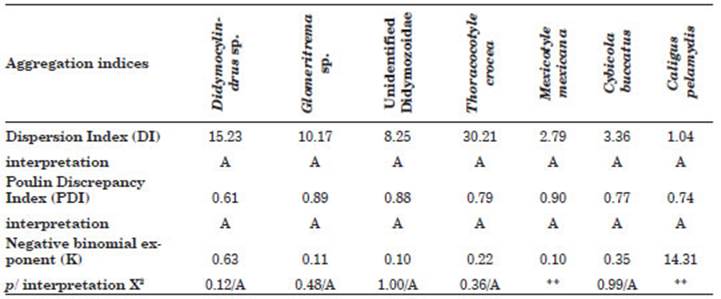
Table 3 Aggregation indices to evaluate the dispersion of the most prevalent ectoparasites in Scomberomorus sierra acquired in Puerto Pizarro, Tumbes, Peru. Abbreviations: p, significance value; X2, Chi-square test value; A, Aggregate. ** Sample too small to verify the fit of the negative binomial distribution.
A negative correlation between the MA and MI of T. crocea, and the TL of the host was found (Table 4). The same situation is observed for the parasite unidentified Didymozoidae. On the other hand, the digenean Glomeritrema sp. is the only species showing a degree of association between the weight of the host and the MA of the parasite. Regarding the host sex factor, this was closely associated with the P% and MA of T. crocea, and for Didymocylindrus sp, sex was as sociated with the MI (Table 4).
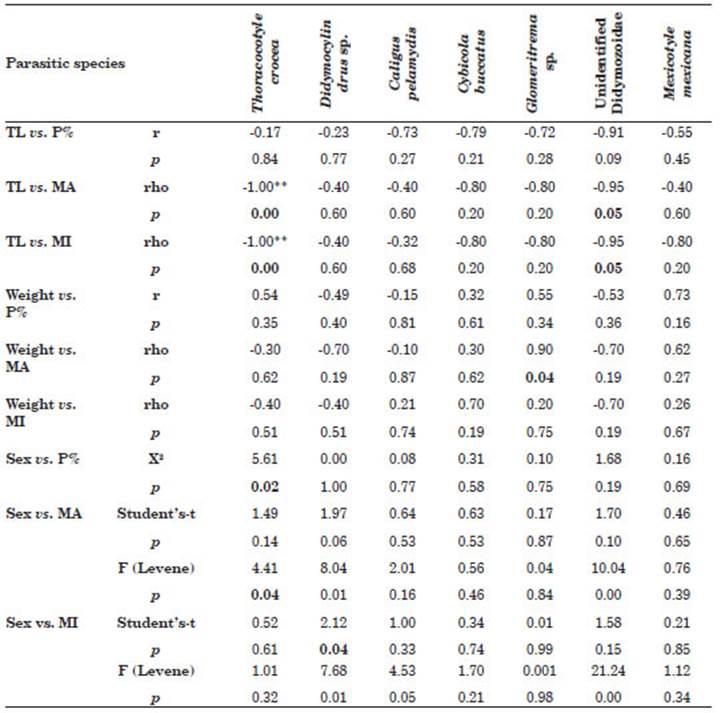
Table 4 Association between total length (TL), weight and sex of Scomberomorus sierra versus prev alence (P%), mean intensity (MI), mean abundance (MA) ectoparasitic acquired in Puerto Pizarro, Tumbes, Peru. Abbreviations: p, significance level; r, Pearson’s correlation; rho, Spearman; X2, Chi-square test; F, Levene test. Values in bold of p indicate statistically significant differences.
There were slight differences according to sex of S. sierra population for the values of the alpha diversity indices (DMn and DMg) of the community component of ectoparasitic metazoans with high er values in females than in males. On the other hand, there were similar values for H, J, D, d and Chao-1 for females compared to males (Table 5).
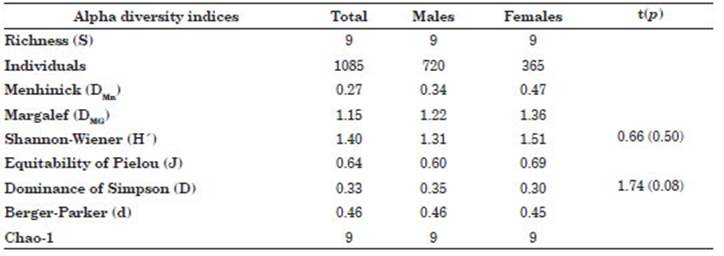
Table 5 Alpha diversity indices for the community component of metazoan ectoparasites according to the sex of Scomberomorus sierra collected in Puerto Pizarro, Tumbes, Peru. Abbreviations: p, sig nificance level; t, Student’s-t; S, Richness; DMn, Menhinick index; DMG, Margalef index; H, Shannon-Wiener index; J, Equitability of Pielou index; D, Dominance of Simpson index; d, Berger-Parker index.
DISCUSSION
The parasitic community of the ectoparasitic metazoan fauna in S. sierra was dominated by seven flatworms and two crustaceans. All were host-specialist ectoparasites (monogeneans, didymozoids and copepods). In the case of ec toparasites in S. sierra, transmission is asso ciated with factors such as habitat, behavior, diet and host density, as well as environmental characteristics (for example, depth and tempera ture) (Poulin, 1995; Barber et al., 2000; Oliva et al., 2004; Miranda‐Delgado et al., 2019; Santos-Bustos et al., 2020). In our research of ectopara sites in S. sierra, the highest P% and the high est frequency of absolute dominance for one species, for two or more species and the relative frequency for ectoparasite species was obtained by the digenean Didymocylindrus sp. followed by the monogenean T. crocea. In the Bay of Mazatlán, Mexico, 11 parasitic species were re corded in S. sierra, of which the highest P% were Didymocylindrus sp. (92%) and Didymocystis scomberomori (MacCallum and MacCallum, 1916) (88%), while the monogenean T. crocea achieved the highest MI value (75.2) (Bárcenas et al., 2021). Didymozoids are also considered to be highly host-specific with the highest frequency of absolute dominance and the relative frequency; this parasite group mainly parasitises tropical and subtropical scombrids. Five didymozoid spe cies have been reported in Scomber japonicusHouttuyn, 1782 from Peru (Cruces et al., 2014). T. crocea has been recorded in all 18 species of Scomberomorus Lacepède (Santos-Bustos et al., 2020). Monogeneans are often transmitted directly between individual hosts through con tact, exhibit high host-specificity, have a direct life cycle and can reproduce in a wide range of temperatures (Santos-Bustos et al., 2020).
The differences observed between the pres ent research and with Santos-Bustos et al. (2020) could be attributed to: (1) host sample size: 59 in our work and 674 in Santos-Bustos et al. (2020); (2) host evaluation period: four years of sampling in this work and in Santos-Bustos et al. (2020) throughout 10 years of monitoring; (3) number of locations evaluated: one in Peru, in the cur rent work, versus four in Mexico (Santos-Bustos et al., 2020); (4) number of biological groups of parasites collected: only three (Trematoda, Monogenea, and Copepoda) in this research, and in contrast in Santos-Bustos et al. (2020) were up to seven, also including Cestoda, Acanthocephala, Nematoda and Isopoda, and finally (5) types of parasites: only ectoparasites in this research ver sus ectoparasites, and endoparasites in Santos-Bustos et al. (2020).
Aggregation is a typical parasite dispersion pattern in marine fishes (Miranda‐Delgado et al., 2019). In all the ectoparasitic species observed in this study, the aggregation indices (DI, PDI, and K) showed a contagious distribution, which is in fluenced by intrinsic and extrinsic factors such as: (a) spatial heterogeneity of the fish habitat that produces differences in susceptibility; (b) influence of the evolutionary history of the para site for food, space and reproductive competition; (c) improvement in the opportunity of infecting fish; and (d) prevention of host population col lapse due to the effects of parasitism (Iannacone et al., 2012).
Regarding the degree of association between parameters, the TL of the host was negatively re lated to the MA and MI of T. crocea and unidenti fied Didymozoidae. No correlation was found be tween the abundance and P% of Didymocylindrus simplex (Ishii, 1935) and the TL of scombrid Katsuwonus pelamis (Linnaeus, 1758) (Justo et al., 2013). Other studies have described a posi tive correlation for the abundance of monoge nean T. crocea in Acapulco, Mexico with the size of the fish (Santos-Bustos et al., 2020). A possible explanation for the fact that the TL of the host was negatively related to the MA and MI of T. crocea and unidentified Didymozoidae. may be that the smaller specimens of S. sierra corre spond to the immature or juvenile forms of the fish, which infers that both species of parasites present a greater burden in juvenile populations. Therefore, it is suggested that the small and ju venile specimens of S. sierra are gregarious and form schools, while the adults of the large species are solitary or less gregarious (Santos-Bustos et al., 2020). For example, it is known that ec toparasitic monogenous populations may be more abundant in schooling fish than in solitary species because the probability of a transmis sion stage (e.g., eggs, larvae) coming into contact with a host increases with a higher host density (Santos-Bustos et al., 2020). In Sphyraena ensis it is known that ectoparasite populations may be more abundant in fish with a schooling behavior than in solitary species because the probability of a transmission stage (e.g., eggs, larvae) con tacting a host increases with greater host density (Minaya et al., 2021b). This pattern is opposite to that observed in other fish species, in which larger fish can facilitate parasite colonization by ecto‐ and endoparasites because larger fish offer a greater surface area for gill‐attaching parasites, tend to ingest larger amounts of food, and are often older, meaning they have had more time to accumulate parasites than smaller, younger individuals (Miranda‐Delgado et al., 2019). Differences in the diet of S. sierra have been reported among the different size classes of these fish (juveniles, pre-adults and adults) (Torres-Rojas et al., 2020).
A close relationship was observed between the sex of the host and the ecoparasitological parameters of T. crocea and Didymocylindrus sp. (Table 4). In other studies, no significant differences were found for the P% and MI of Didymocylindrus filiformisIshii, 1935 between the sexes of scombrid K. pelamis (Silva et al., 2017). Our results are surprising, because bio logical differences between males and females of S. sierra have not previously been reported in the literature (Aguirre-Villaseñor et al., 2006; Lucano et al., 2011; Espino-Barr et al., 2017; Justo & Kohn, 2014; Torres-Rojas et al., 2020). Thus, there is a clear indication of the prefer ence of the parasites for one of the sexes of the host fish. However, all that has been published to date in the scientific literature does not show differences in the ecological relationships (habi tat, behavior, and feeding) of males and females of S. sierra and does not help us to adequately explain the differences found between the sex of the host and the ecoparasitological parameters of T. crocea and Didymocylindrus sp. (Sandoval-Ramírez et al., 2020; Torres-Rojas et al., 2020). This interpretation would also apply the differ ences observed for the parasitological indices of T. crocea and Didymocylindrus sp. and for DMn and DMg (Iannacone et al., 2013; Rey et al., 2022). It should be remembered that for ectoparasites of S. sierra, S, H, J, D, d and Chao-1 did not show differences between sexes of hosts. The Chao-1 index shows that the size of the host sample was adequate.
In Peru, the monogeneans T. crocea and M. mexicana are species that have been reported in S. sierra, T. crocea from Trujillo in northern Peru (Tantaleán et al., 1985; Luque et al., 2016) and M. mexicana from Chimbote in northern Peru (Tantaleán et al., 1988; Luque et al., 2016). Likewise, the presence of these two monoge neans has also been recorded in Mexico Lamothe et al., 1997; Pérez et al., 1999; Mendoza-Garfi et al., 2017; Bárcenas et al., 2021). The finding of both species of monogeneans in both Peru and Mexico confirms their specificity towards S. sier ra (Costello, 2016). In Mexico, T. crocea reached the highest MI levels probably related to the anomalous warm conditions since S. sierra were collected during the “El Niño” event and mono geneans may be more abundant during times of prolonged heat (Bárcenas et al., 2021).
The digeneans of the Didymozoidae fam ily, are considered scombrid specialists and are transmitted by ingestion (Pozdnyakov & Gibson, 2008). They are a group with high P% that is part of the helminthofauna of S. sierra (Bárcenas et al., 2021). Digeneans of the family Didymozoidae are parasites of marine fish with high host specificity. They mainly parasitize tropical and subtropical scombrids (Miranda‐Delgado et al., 2019). The high vagility and endothermy of scombrids require the need for high metabolic energy which is met by foraging large quanti ties of food items (e.g., crustaceans, fish of the Engraulidae and Clupeidae families, molluscs, and finally, polychaetes) and can function as in termediate and paratenic hosts for didymozoids. This indicates that this species act as tertiary consumers, or mesopredators, in the food net work (Miranda-Delgado et al., 2019; Sandoval-Ramírez et al., 2020). The high prevalence and medium abundance levels of Didymocylindrus sp., could be explained by the preference of S. sierra to prey on fish (Moreno et al., 2011), since these actinopterygians are intermediate or paratenic hosts for didymozoids of this group of trematodes (Bárcenas et al., 2021). In northern Peru, S. sierra has a record for a representative of the Didymozoidae family, which is the species Didymozoon sp. (Tantaleán et al., 1992; Luque et al., 2016) that were also recorded in the present study.
In the Mexican Pacific, two species of flukes of the Bucephalidae family have been recorded as parasitizing S. sierra: Bucephalus heterotentacu latusBravo and Sogandares, 1956 in the state of Guerrero and Prosorhynchoides cybii (Park, 1939) in Colima (Bravo & Sogandares, 1956), while in northern Peru, Anaporrhutum sp. a digenean of the Gorgoderidae family (Tantaleán et al., 1992; Luque et al., 2016) has been described. However, in the present study no members of either the Bucephalidae family or the Gorgoderidae family were found. This could be explained by the varia tions in abiotic factors such as local fluctuations in surface temperature are known to affect many ecological processes, including the productiv ity of food webs, and the transmission rates of many trophically transmitted endoparasites as Bucephalidae family and Gorgoderidae family due to population decreases in their potential in termediate hosts (Santos-Bustos et al., 2020).
On the Pacific coast of Mexico, 24 parasitic metazoan taxa which were collected from four sampling sites were determined in S. sierra (3 monogeneans, 8 trematodes, one cestode, one acanthocephala, 4 nematodes, 5 copepods, and 2 isopods). Trematodes presented the highest rich ness, representing 34% of the total species re covered, followed by copepods (29%). According to the infection site, 13 species of parasites were classified as ectoparasite and 11 as endopara sites (Santos-Bustos et al., 2020). The present study also presented a greater richness in trema todes, which represented 57% of the total species found. However, according to the infection site, all the specimens recovered in this study were ectoparasites, since they were all collected from the gills of their hosts. These results could suggest that there are variations in local environmental factors of Peruvian marine waters (i.e., tempera ture, salinity and other abiotic factors) that have a substantial effect on community structure of important intermediate potential hosts for intes tinal helminths endoparasites (Santos-Bustos et al., 2020).
Copepods are the second largest parasitic group in marine fish. Members of the Caligidae family being the most frequent ectoparasites, due to their ability to swim and move (Morales et al., 2016). Apparently, crustaceans are neither abun dant nor frequent in scombrid fish (Miranda-Delgado et al., 2019). Only two species have been recorded in the parasite communities of S. sierra. A study carried out in Veracruz, Mexico recorded the copepods C. pelamydis and C. buccatus in the fish Scomberomorus cavalla (Cuvier, 1829) (Villar-Beltrán et al., 2019). In our study, S. si erra presented both copepods, which suggesting a possible affinity for the Scombridae family. Likewise, C. pelamydis has not previously been recorded in S. sierra, and this is the first study to report this association. In Peru, the copepods Acantholochus nudiusculus (Cressey & Cressey, 1980), Caligus omissus Cressey & Cressey, 1980 and C. buccatus (Luque et al., 2016) have been previously recorded.
As suggested by Bárcenas et al. (2021), we agree that two main factors seem to deter mine the structuring of the parasitofauna of S. sierra: the food chain network and the phylogenetic affinities of certain groups of helm inths (i.e., Didymozoidae at a family level and Thoracocotylidae at a host genus level). The nine species recorded in S. sierra in the present study are considered specialist species as they are only present in the Scombridae family (Santos-Bustos et al., 2020).
CONCLUSIONS
In conclusion, the ectoparasitic metazoan community of S. sierra was made up of mono geneous specialist ectoparasites, didymozoid flukes, and copepods, which are taxa of parasites common to various species of scombrids, and of these only T. crocea, Didymocylindrus sp. and unidentified Didymozoidae. showed dependence (positive and negative) on the morphological parameters of their host. Didymocylindrus sp., Glomeritrema sp., unidentified Didymozoidae, S. scomberomori, and C. pelamydis are five new re cords of parasites in S. sierra for Peru.












 uBio
uBio 

