Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.113 no.2 Cap. Fed. jun. 2021
http://dx.doi.org/10.25132/raac.v113.n2.eras04wm.ei
Articles
ERAS® in Colorectal Surgery
1 Miembro investigador del Departamento de Cirugía General, Royal Surrey County Hospital, Guildford, Reino Unido.
2 Profesor Consultor en Cirugía Colorrectal, Royal Surrey County Hospital, Guildford, Reino Unido
Introduction
Enhanced Recovery After Surgery (ERAS®) is an evidence-based multimodal perioperative protocol focused on stress reduction and the promotion of a return to function1. It now covers all major abdominal operations along with head and neck, cardiac and thoracic surgery. The first ERAS® protocol was published and implemented by Fearon et al. in 2005 for colonic resections2. Since then, the use of ERAS® has been most extensively studied in colorectal surgery1. The most recent published guidance for elective colorectal surgery was released as a fourth edition in the World Journal of Surgery by Gustafsson et al. in 20183.
Prior to ERAS®, surgical groups reported their own ‘fast track’ or enhanced recovery programmes with wide variations in design2. They involved optimising pain relief, stress reduction with regional anaesthesia, early enteral nutrition and early mobilisation4,5. These factors helped demonstrate improvements in physical performance, pulmonary function, body composition and a marked reduction of length of stay4-6. However, due to the sporadic implementation of these elements, there was considerable variation in measured rates of recovery and length of stay2.
The notion of formalised collaboration to improve perioperative care by standardisation was conceived in 2001 by Professors Ken Fearon and Olle Ljungvist at a London nutrition symposia7. At the time, the ERAS® Study Group was formed, which later matured into the ERAS® Society with the mission to improve outcomes for patients undergoing surgery by education, scientific work, and implementation of evidence-based guidelines8.
From its origins in Europe, international collaboration for ERAS® has facilitated its reaches globally9. The ERAS® Society established early on that protocols alone were not enough for implementation away from traditional care10. They appointed Centres of Excellence which requires completion of an ERAS® implementation programme, qualifying as a teaching centre for the implementation programme, and/or making considerable contributions to the development of ERAS®. The Latin America branch of the society, known as ERAS® LatAm, covers the countries of South America and the Caribbean. In October 2017, an inaugural meeting in Montevideo, Uruguay was arranged and led by Professor Adrian Alvarez where ERAS® LatAm was established.
The scope of the ERAS® guidelines for colorectal surgery has broadened significantly and now benefits patient care by well-established published evidence1,11. Indeed, ≥70 % compliance to the ERAS interventions has shown a risk reduction of 5-year cancer-related death by 42%12. There are many criteria used in the concept of “marginal gains” that weave in a complex interplay across the perioperative pathway for patients. The four headings, which encompass the patient journey from diagnosis to recovery are pre-admission, pre-operative, intra-operative and post-operative3. This article summarises these headings and discusses the latest interventions recommended by the ERAS® Society for colorectal surgery.
Pre-admission
Recently, there has been a greater emphasis on the preparatory phase of care prior to undertaking surgery. Risk assessment and patient optimisation are important components of a patient’s care3. Latest interventions include anaemia correction and prehabilitation with nutritional support.
Risk Assessment
Risk assessment of patients serves several important functions:
I. Identification of pre-existing comorbidities and potential improvement of these prior to surgery
II. Accurate risk stratification allows for improved patient understanding and informed consent
III. Provision of services and resources (including the availability of higher dependency post-operative care)
IV. Audit and assessment of surgical performance against predicted standards
V. Provision of an appropriate forum to undertake detailed information sessions for the patient
VI. Identification of patients that are either unsuitable for or unwilling to undertake operative management
Patient assessment prior to surgery should employ a multidisciplinary approach. Whilst current guidelines do not dictate how to undertake such assessments, it is widely accepted that a thorough medical history and clinical examination combined with appropriate use of investigation is mandatory13. Risk stratification tools are essential and can help identify those at most risk, such as those with malnutrition for nutritional support or sarcopenic patients for targeted exercise programmes.
Optimisation
In the period between diagnosis and the date of surgery there is a window to address the modifiable risk factors and influence beneficial lifestyle changes. Sub-specialty opinion on chronic disease management including heart disease, respiratory disease, kidney disease, hypertension and diabetes should be undertaken to ensure optimisation prior to surgery.
Patients should be encouraged to stop smoking. Current smokers are three to six times more likely to suffer a pulmonary complication and smoking cessation prior to surgery has been demonstrated to reduce this risk as well as improve wound healing14,15. Equally, while the evidence base for alcohol avoidance is less developed, studies do suggest that excess alcohol consumption (defined as more than 2 units of alcohol a day) may be associated with increased rates of post-operative infections16.
Anaemia is common in patients undergoing colorectal surgery and can be attributed to luminal bleeding, Vitamin B12 and folate deficiency or the result of chronic disease3. There is association with significant morbidity and mortality. Furthermore, perioperative transfusions are associated with surgical site infection, septic shock and have an adverse effect on overall survival in a colorectal cancer cohort17. Correction of anaemia can be effectively and safely achieved using intravenous iron negating the need for blood transfusion3.
Prehabilitation
Prehabilitation is a multimodal intervention, aimed at augmenting an individual’s functional capacity in anticipation of a forthcoming stressor. It may be likened to an athlete training for competition and includes nutritional optimisation, physical exercise programmes and targeted psychological intervention.18
The ERAS® society recommend prehabilitation exercise programmes that target:
I. Aerobic capacity
II. Muscle strength and endurance
III. Daily physical activity
Studies employing such programmes and incorporating nutritional and psychological interventions have demonstrated improved physiological reserve prior to surgery and sustained post-operative functional capacity19 (Fig. 1). It is recommended that such programmes are designed, delivered, and supervised by a trained appropriate medical professional and are specifically tailored to an individual’s functional status and capacity20.
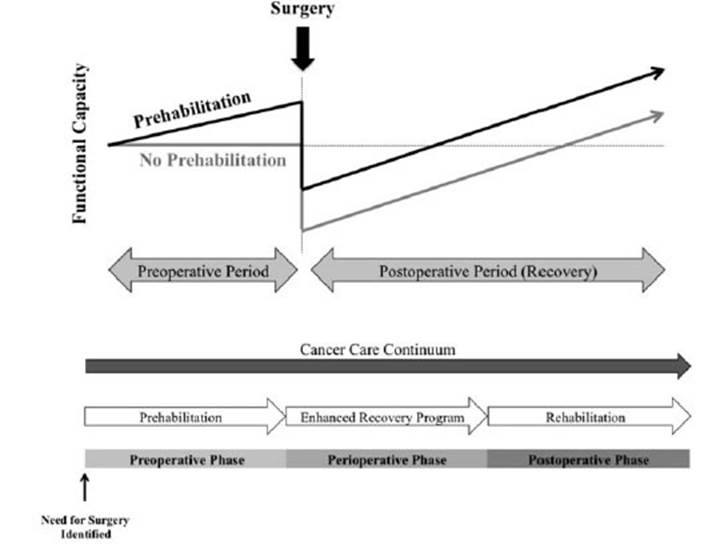
Figure 1 Trajectory of functional capacity in the cancer care continuum showing sustained advantages into the postoperative period from prehabilitation (Minnella et al. 2017)19.
Malnutrition results in increased morbidity, mortality and poor oncological outcomes, yet 25- 40% of patients are malnourished on admission to hospital21. Nutritional interventions commenced prior to surgery have been demonstrated to reduce risk of post-operative infection, reduce rates of anastomotic leak and shorten length of stay22,23.
Evidence for improved outcomes following acute psychological intervention (including relaxation exercises, role play and stress management techniques) is less well established, but such interventions show improved quality of life outcome measures, depression scores and body image assessments24.
Prehabilitation is an intervention that is in its nascency and the science is continually evolving. In particular, the greatest potential benefit comes to patients who are most at risk and it is crucial that further work is done to explore such programmes.
Pre-operative
This section discusses the pharmacological considerations to take prior to anaesthesia on admission to hospital.
Antiemetics
Post-operative nausea and vomiting (PONV) are both very common - 30% and 50% respectively for all surgical patients3. It may result in dehydration, delayed return of adequate nutrition intake, or it may necessitate the placement of a nasogastric tube, increased intravenous fluid administration post-operatively, prolonged hospital stay, and increased healthcare costs3. The causes are multifactorial and these can be categorised into patient, anaesthesia and surgical-related factors. Those at more risk of developing PONV should be established at the pre-operative risk assessment and up to 3 multimodal antiemetics should be used in those with ≥ 2 risk factors3,25.
Anxiolytics
Evidence suggests that pre-operative education addresses the issue of psychological distress prior to surgery and can bring patient anxiety to an acceptable level to avoid the need for anxiolytic medication26. The use of routine sedative medications should be avoided as side-effects from opioids, beta blockers and especially benzodiazepines are shown to be detrimental. The ERAS® guidance suggests the use of opioid sparing pre-anaesthetic drugs in a multimodal fashion such as acetaminophen, NSAIDS and gabapentinoids.3
Antimicrobial Therapy and Mechanical Bowel Prep
These two items are considered together as studies have incorporated the use of antibiotic prophylaxis combined with mechanical bowel prep (MBP) as a means to reduce bacterial load. Antibiotics both in systemic and oral form reduce the risk of surgical site infection from 39 to 13%27. Consideration should also be given to the gut microbiome which is thought to play an integral role in post-operative ileus and anastomotic leak, newer studies are addressing the potential advantages that antibiotics confer towards this28.
The ERAS® recommendation is to use a cephalosporin in combination with metronidazole, systemic antibiotics should be given as a single dose 60 minutes prior to incision3. The use of antibiotics in the oral form are recommended only when MBP is given. MBP alone in colonic surgery is of no benefit and may have potential negative effects in terms of fluid and electrolyte disturbance prior to surgery, as well as being unpleasant28. Practically, there may be some benefit of MBP in rectal surgery, which will avoid stool remaining in a diverted colon if a defunctioning ileostomy is planned. A rectal enema, may be just as efficient for this and carries little or none of the risks of MBP28.
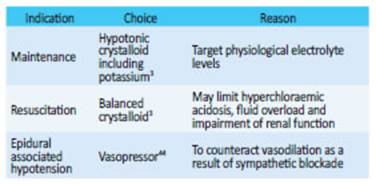
Fluid Assessment
Fluid and electrolyte levels should be assessed throughout the perioperative period. A patient should arrive at theatre in a euvolaemic state. Prolonged fasting preceding surgery can be avoided and patients should be allowed to drink non-alcoholic clear fluids up to 2 hours before and a light meal should be allowed up to 6 hours before3. If MBP is used then this may incur a negative fluid balance by as much 2L - intravenous fluid therapy to correct may be warranted.
Carbohydrate drinks improve pre-operative well-being, reduce post-operative insulin resistance, decrease protein breakdown and better maintain lean body mass and muscle strength, as well as beneficial cardiac effects3,29. They come as a clear fluid and can therefore be taken up to 2 hours prior to surgery.
Intra-operative
Standard Anaesthetic Protocol
Choice and use of anaesthetic agents will impact the early post-operative period. Propofol allows for rapid awakening and one should avoid benzodiazepines and only use the short-acting class of opioids if required to reduce post-operative delirium.3 Evidence is limited, but there is a high recommendation to use total intravenous anaesthesia (TIVA) with Propofol, as anaesthetic gases may increase PONV. The use of the bi-spectral index for cerebral function monitoring will reduce the risk of awareness and avoid overdose and delirium, which is a particular risk in the elderly30.
Deep neuromuscular blockade allows the surgeon to operate with lower intra-abdominal pressures. Careful monitoring is required with acceleromyography as poor reversal can increase the risk of pulmonary complications from residual paralysis3. Higher pressures will worsen cardiac function, impede ventilation and reduce renal blood flow31. Intrabdominal pressures as low as 8mmHg can be achieved and this is associated with a physiological improvement that leads to faster recovery, fewer intraoperative complications and less inflammation32.
Surgical Access
Perhaps the greatest change since the concept of ERAS® is the increased use of minimally invasive surgery (MIS). Colorectal resections are now well-established in the form of MIS and it is the standard of care in many countries3. The majority of evidence is in the comparison of laparoscopic versus open surgery. Three Cochrane systematic review articles have demonstrated that laparoscopy confers advantages to recovery, length of stay, blood loss and complications for colonic and rectal resections33-35. Oncological recurrence rates did not show any significant differences34,35. A large national audit in Holland, covering 2010-2013, revealed that laparoscopic resection had significantly lower 30- day mortality36.
More recently, advanced MIS techniques for total mesorectal excision have been taken up in the guise of robotics and transanal approach (TATME). The focus for the different MIS formats is in improving cancer-related outcomes, reducing morbidity of pelvic surgery and reducing conversion rates3. A systematic review from 2019 of 29 RCTs and meta-analysis of 6237 patients compared all four modalities for rectal resections (open, laparoscopic, robotic and TATME). It concluded that laparoscopic and robotic surgery may improve post-operative recovery, but open and transanal approach may gain better oncological resection37. The evidence for these two newer techniques of robotic and TATME approaches is still in the early stages, and the paper itself admits to areas of bias across the board. The meta-analysis only had 50 patients in the TATME arm and actually a recent review from Norway worryingly revealed a more than double rapid recurrence pattern in 10% of patients38.
The newer equipment required for both robotic surgery and TATME is expensive with higher running costs per case and the cost-effectiveness vs the current standard of care in laparoscopy is a crucial aspect for consideration39.
Ultimately, no one form of MIS is yet to demonstrate clear superiority, but MIS in itself is strongly recommended by ERAS®. MIS enables many of the elements for ERAS® and is an independent predictor of good outcome by reduced pain, early mobilization, less impact on fluid shifts and reduced ileus3. Ultimately, the outcome is not purely down to the method chosen, but it is individual surgeon’s technical ability to use that method. Good surgery will minimise tissue trauma and achieve the operation in a timely, yet safe manner to reduce immediate complications and surgical stress response.
Post-operative
Post-operative care should be seen in continuum with peri-operative management, and the cornerstone remains in limiting the surgical stress response and encouraging a swift return to normal activities. Patients should be educated on what to expect during the post-operative period along with their goals of management, including early mobilisation, meeting nutritional requirements and timely removal of indwelling tubes.
Analgesia
Multimodal post-operative analgesia, achieving adequate pain control with the avoidance of opiates, is fundamental to the ERAS® approach. This enables early mobilization and is associated with a faster return of bowel function, reduced morbidity and shorter length of stay3.
Paracetamol and non-steroid anti-inflammatory drugs (NSAIDs) should be routinely used. Although there are concerns for association of NSAIDs with anastomotic leak, this data remains unclear and so guidelines currently support their use. Some research is now focusing on selective NSAIDs, which may prove a useful adjunct40. There are many other options under consideration, including lidocaine infusion, dexmedetomidine and ketamine, however the optimum approach is not yet defined.
Thoracic epidural, containing local anesthetic and lipophilic opioid, was the gold standard in the era of open surgery; associated with reduced cardiopulmonary complications, ileus and mortality41. However, with the widespread implementation of minimally invasive colorectal surgery there has been a shift of focus. Technical issues have become more relevant as outcome measures are no longer superior, these include failure and lumbar placement, increasing risk of urinary retention and lower limb paralysis42. Thoracic epidural anaesthesia may still be considered where there is a high risk of conversion; but is largely superseded by other adjuncts such as spinal anaesthesia, lidocaine infusion and local wound infiltration. Transversus abdominus plane (TAP) blocks, targeted between the transversus abdominus and internal oblique muscles, is one adjunct that has been widely implemented. They are limited by a relatively short half-life and act only for incisions below the level of the umbilicus, but may reduce opiate consumption and the risk of urinary retention43.
Post-operative fluid and electrolyte therapy
There is not universal consensus on post-operative fluid therapy, however the aim is to maintain a balance close to zero. Oral fluid should be encouraged when patients are awake and free of nausea, and intravenous fluid administration stopped on the day of surgery. It is important to recognise when patients are hypovolemic or unable to maintain adequate fluid intake, so that supplementary fluids can be considered as outlined on Table 1. When evaluating fluid status, oliguria should be considered with caution: it can result from the surgical stress response and should not be an indication for fluid resuscitation alone.
Nutritional Care
Historically, after colorectal resection patients were starved for extended periods with routine nasogastric tube placement. This is against the principles of the ERAS® approach and no longer followed: nasogastric tubes should not be routinely used, and a Cochrane review demonstrates that early oral feeding is not only safe but may be associated with reduced morbidity.45 If tolerated, nutritional supplements can be prescribed from the day of surgery to minimize the negative protein and energy balance, and immunonutrition should be considered in malnourished patients46. Despite initial concerns, early oral nutrition is not associated with increase in ileus. Data now shows that an established ERAS® protocol, including all these elements described, is the most effective measure to prevent ileus. Interventions that do not cause harm, for example chewing gum and coffee, can be considered but have no proven efficacy3,47. The ERAS® protocol also limits post-operative hyperglycemia, through a reduction in the surgical stress response and associated insulin resistance. However, in cases of persisting post-operative hyperglycaemia, insulin can be considered to reduce infective complications.
Urine Drainage
Early catheter removal is associated with lower rates of urinary retention and recommended to enable early mobilisation and reduce the risk of urinary tract infection48. Selective delayed removal to the 2nd or 3rd day may be applied to higher risk groups, including rectal surgery, male patients and those with epidural anaesthesia49.
Thromboprophylaxis
It is well established that all surgical patients should receive mechanical and pharmacological thromboprophylaxis while in hospital to reduce the risk of deep vein thrombosis. Current data supports the administration of extended (28 day) low molecular weight heparin after resection of abdominal and pelvic cancer50. It is not clear whether this benefit will confer to laparoscopic surgery and so this recommendation may change.
Conclusion
The ERAS® guidance for colorectal surgery is becoming better established globally. It can only be achieved by a multidisciplinary approach to incorporate the wide variety of marginal gain aspects for the individual patient. The guidance is deep rooted, but new technology and evidence continues to emerge. Therefore, expansion and changes to the protocol recommendations will continue in line with consensus from international collaboration and high-quality research outcomes.
Referencias bibliográficas /References
1. Pędziwiatr M et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. 2018;35. [ Links ]
2. Fearon KCH, et al. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin. Nutr. 24, 466-477 (2005). [ Links ]
3. Gustafsson UO et al. Guidelines for Perioperative Care in Electi ve Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J. Surg. 2019;43, 659- 95. [ Links ]
4. Basse L, Jakobsen DH, Billesbølle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann. Surg. 2000;232: 51-7. [ Links ]
5. Basse L, et al. Accelerated postoperative recovery programme af ter colonic resection improves physical performance, pulmonary function and body composition. Br JSurg. 2002;89, 446-53. [ Links ]
6. Wind J, et al. Systematic review of enhanced recovery program mes in colonic surgery. Br. J. Surg.2006; 93: 800-9. [ Links ]
7. ERAS® Society Webpage. Available at: Available at: https://erassociety.org/ about/history/ . (Accessed: 1st May 2020) [ Links ]
8. Sosa JA, Ljungqvist O. World Journal of Surgery Becomes the Offi cial Publication of the ERAS Society. World J Surg. 2018;42:2689- 90. [ Links ]
9. Joliat G R, Ljungqvist O, Wasylak T, Peters O, Demartines N. Beyond surgery: Clinical and economic impact of Enhanced Recovery after Surgery programs. BMC Health Serv Res 2018; 18:1-4. [ Links ]
10. Maessen J, et al. A protocol is not enough to implement an en hanced recovery programme for colorectal resection. Br J. Surg. 2007;94:224-31. [ Links ]
11. Ljungqvist O. ERAS - Enhanced Recovery after Surgery: Moving Evidence-Based Perioperative Care to Practice. J Parenter Enter Nutr.2014; 38:559-66. [ Links ]
12. Gustafsson UO, Oppelstrup H, Thorell A, Nygren J, Ljungqvist O. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J Surg. 2016;40: 1741-7. [ Links ]
13. Rafferty JF. Preoperative Management. In: The ASCRS Textbook of Colon and Rectal Surgery 125-135 (New York: Springer; 2011). doi:10.1007/978-1-4419-1584-9_8 [ Links ]
14. Mills E, et al. Smoking cessation reduces postoperative com plications: A systematic review and meta-analysis. Am J Med. 2011;124:144-54. [ Links ]
15. Thomsen T, Villebro N, Møller AM. Interventions for preope rative smoking cessation. Cochrane Database Syst Rev. 2014. doi:10.1002/14651858.CD002294.pub4 [ Links ]
16. Shabanzadeh DM, Sørensen LT. Alcohol consumption increases post-operative infection but not mortality: A systematic review and meta-analysis. Surg Infect (Larchmt). 2015;16:657-68. [ Links ]
17. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients under going colorectal cancer surgery: A systematic review and meta-analysis. Ann Surg.2012;256:235-44. [ Links ]
18. Carli F, et al. Randomized clinical trial of prehabilitation in colorec tal surgery. Br J Surg. 2010;97:118797. [ Links ]
19. Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta Oncol (Madr). 2017;56: 295-300. [ Links ]
20. Minnella EM, Gillis C, Edgar L, Carli F. Prehabilitation. In: Enhanced Recovery After Surgery. 89-99 Springer International Publishing; 2020. doi:10.1007/978-3-030-33443-7_10 Falta ciudad de publi cación. [ Links ]
21. Bozzetti F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition.2002; 18:953- 9. [ Links ]
22. Gillis C, et al. Prehabilitation versus rehabilitation: A randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014; 121:937-47. [ Links ]
23. Waitzberg DL, et al. Postsurgical infections are reduced with spe cialized nutrition support. World J. Surg. 2006;30:1592-604. [ Links ]
24. Tsimopoulou I, et al. Psychological Prehabilitation Before Cancer Surgery: A Systematic Review. Ann Surg Oncol . 2015;22:4117- 23. [ Links ]
25. Eberhart LHJ, Mauch M, Morin AM, Wulf H, Geldner G. Impact of a multimodal anti-emetic prophylaxis on patient satisfaction in high-risk patients for postoperative nausea and vomiting. Anaesthesia. 2002; 57:1022-7. [ Links ]
26. Wilson CJ, et al. Caring for the surgically anxious patient: a review of the interventions and a guide to optimizing surgical outcomes. Am J Surg. 2016;212: 151-9. [ Links ]
27. Nelson RL, Gladman E, Barbateskovic M. Antimicrobial pro phylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014; 2014. [ Links ]
28. Singh R, et al. Future perspectives: enhanced recovery in co lorectal surgery. Anaesthesia.2020;75: e14-17. doi:10.21037/ales.2020.03.07 [ Links ]
29. Gianotti L, et al. Preoperative Oral Carbohydrate Load Versus Placebo in Major Elective Abdominal Surgery (PROCY). Ann Surg. 2018;267:623-30. [ Links ]
30. Chan M TV, Cheng BCP, Lee T. MC, Gin T. BIS-guided anesthesia de creases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33-42. [ Links ]
31. Demyttenaere S, Feldman LS, Fried GM. Effect of pneumoperito neum on renal perfusion and function: A systematic review. Surg Endosc Other IntervTech. 2007;21:152-60. [ Links ]
32. Díaz-Cambronero O, et al. Effect of an individualized versus stan dard pneumoperitoneum pressure strategy on postoperative re covery: a randomized clinical trial in laparoscopic colorectal sur gery. Br JSurg. 2020. doi:10.1002/bjs.11736 [ Links ]
33. Schwenk W, Haase O, Neudecker JJ, Müller JM. Short term bene fits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005. doi:10.1002/14651858.cd003145.pub2 [ Links ]
34. Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochra ne Database Syst Rev. 2008. doi:10.1002/14651858.CD003432.pub2 [ Links ]
35. Vennix S, et al. Laparoscopic versus open total mesorectal exci sion for rectal cancer ( Review ) Summary of findings for the main comparison. 2014. doi:10.1002/14651858.CD005200.pub3 www.cochranelibrary.com [ Links ]
36. Gietelink L, et al. Reduced 30-day mortality after laparoscopic co lorectal cancer surgery. Ann. Surg. 2016;264:135-40. [ Links ]
37. Simillis C, et al. Open Versus Laparoscopic Versus Robotic Versus Transanal Mesorectal Excision for Rectal Cancer: A Systematic Review and Network Meta-analysis. Ann Surg. 2019;270:59- 68. [ Links ]
38. Larsen SG, Pfeffer F, Kørner H. Norwegian moratorium on transa nal total mesorectal excision. Br. J. Surg. 2019;106:1120-1. [ Links ]
39. Khan JS, Banerjee A K, Kim SH, Rockall TA, Jayne DG. Robotic rectal surgery has advantages over laparoscopic surgery in selected pa tients and centres. Color Dis. 2018. doi:10.1111/codi.14367 [ Links ]
40. Modasi A, Pace D, Godwin M, Smith C, Curtis B. NSAID adminis tration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg. Endosc. 2019;33:879- 85. [ Links ]
41. Pöpping DM, et al. Impact of epidural analgesia on mortality and morbidity after surgery: Systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2014;259:1056-67. [ Links ]
42. Carli F, et al. Evidence basis for regional anesthesia in multidisci plinary fast-track surgical care pathways. Reg Anesth Pain Med. 2011;36:63-72. [ Links ]
43. Torgeson M, Kileny J, Pfeifer C, Narkiewicz L, Obi S. Conventional Epidural vs Transversus Abdominis Plane Block with Liposomal Bupivacaine: A Randomized Trial in Colorectal Surgery. J Am. Coll Surg. 2018;227:78-83. [ Links ]
44. Holte K, et al. Epidural Anesthesia, Hypotension, and Changes in Intravascular Volume. Anesthesiology 2004;100:281-6. [ Links ]
45. Andersen HK, Lewis SJ, Thomas S. Early enteral nutrition within 24h of colorectal surgery versus later commencement of feeding for postoperative complications. Cochrane Database Syst Rev. CD004080 (2006). doi:10.1002/14651858.CD004080.pub2 [ Links ]
46. Smedley F, et al. Randomized clinical trial of the effects of preope rative and postoperative oral nutritional supplements on clinical course and cost of care. Br. J. Surg. 2004;91:983-90. [ Links ]
47. de Leede EM, et al. Multicentre randomized clinical trial of the effect of chewing gum after abdominal surgery. Br J Surg. 2018;105:820-8. [ Links ]
48. Grass F, et al. Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surg Res. 2017;207:70-6. [ Links ]
49. Zmora O, Madbouly K, Tulchinsky H, Hussein A, Khaikin M. Urinary bladder catheter drainage following pelvic surgery - Is it necessary for that long? Dis Colon Rectum 2010;53:321-6. [ Links ]
50. Felder S, et al. Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Data base Sist Rev. 2019;3(3). doi:10.1002/14651858.CD004318.pub5. www.cochranelibrary.com [ Links ]











 texto em
texto em