Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.113 no.2 Cap. Fed. jun. 2021
http://dx.doi.org/10.25132/raac.v113.n2.1494.ei
Introduction
Appendiceal mucinous neoplasm (AMN) was described in 1842 by Rokitansky as a luminal dilation of the appendix caused by accumulation of mucus secretion1,2.
Non-carcinoid appendiceal neoplasms have an incidence of about 0.9 per 100,000 per year; thus, AMNs represent 0.2-0.7% of all appendectomies1,3,4, while 25% correspond to adenocarcinomas.
These tumors are more prevalent in women (female-to-male ratio 4:1) with mean age of 55 years5.
Incidental finding is the most common clinical presentation, but they may present as chronic pain in the right iliac fossa or be found in appendectomy specimens sent for pathological examination3,5,6. The diagnosis should be suspected in patients with chronic pain in the right iliac fossa1,7.
Non-carcinoid appendiceal neoplasms include adenoma (similar to colorectal adenoma), serrated lesion (polyp), low-grade appendiceal mucinous neoplasms (LAMN), high-grade appendiceal mucinous neoplasms (HAMN), mucinous adenocarcinoma, nonmucinous appendiceal adenocarcinoma 4,8-10.
The World Health Organization (WHO) classifies LAMNs as non-invasive tumors7. High-grade appendiceal neoplasms share some histological features with LAMNs but exhibit more aggressive cytologic atypia and are less prevalent8,10. Both LAMNs and HAMNs are considered benign neoplasms as they do not produce infiltrative invasion like mucinous adenocarcinoma4. These tumors have malignant potential as they may perforate and spread throughout the peritoneal cavity. Perforation may be spontaneous or iatrogenic, and when it includes abundant mucin production, the term pseudomyxoma peritonei (PMP) is used. This serious complication may cause mucinous ascites and peritoneal implants2,4,6,9.
KRAS mutation is common but microsatellite instability or BRAF mutation are rare6,8,10,11.
Appendicular mucocele is a descriptive term used to encompass benign or malignant conditions that can produce cystic dilatation of the appendix3,6; or may just be a retention cyst (inflammatory mucocele)3. Lesions > 20 mm are more likely to be mucinous neoplasms, especially in the absence of obstruction5,6. Mucoceles < 20 mm are rarely malignant, but those > 60 mm have high probability of malignancy and spontaneous perforation1,12.
Preoperative diagnosis is difficult in the absence of luminal dilation, but it should be suspected if the intraluminal diameter of the appendix is > 13 mm or if there are mural calcifications or intraluminal contents3,14.
Accurate diagnosis of AMN and PMP is clinically relevant as management may include prolonged follow-up or radical treatment such as cytoreductive surgery with intraperitoneal chemotherapy6,8.
Therefore, appendectomy is the treatment of choice for AMN to preserve the integrity of the surgical specimen. Management in case of positive margins with cells or mucin is controversial. Some authors suggest extending the resection while others state that follow-up is safe even in case of perforation15. Right hemicolectomy does not offer any additional benefit over appendectomy alone for patients with LAMN8,10. Unnecessary incisions in the entire wall of the appendix should not be made during surgery to prevent dissemination of mucus or neoplastic cells into the peritoneal cavity13,16.
There is little evidence about the postoperative management and follow-up of neoplasms of the appendix. A reasonable strategy includes contrast-enhanced computed tomography (CT) scan of the abdomen and tumor markers (CEA, CA 125, CA19.9) one year after the resection, decreasing the frequency in case of normal results4, discontinuing follow-up after 5 years6,9,13,17,18.
Colonoscopy is recommended to rule out synchronous neoplasms4,6,20.
Objectives
The aim of this study was to present four case reports of patients admitted in the emergency department of Sanatorio Parque (Centro de Emergencia Rosario) with acute appendicitis who underwent appendectomy at the Department of General Surgery during 2019 and had a histopathological diagnosis of NMA.
Case reports
We describe four case reports. The demographic and laboratory data are described in Table 1.
All the patients underwent contrast-enhanced multislice CT scan of the abdomen and pelvis to rule out other diagnoses of acute abdomen. The history of iodinated contrast media adverse reactions and kidney function were evaluated before the procedure and beta subunit was determined in all women of childbearing age before exposure to radiation.
Case #1
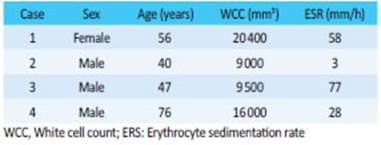
A 56-year-old female patient without significant history sought medical care due to periumbilical abdominal pain that had started five days before and radiated to the right iliac fossa, accompanied by fever within the past 24 hours. On physical examination, she presented pain in the right iliac fossa and hypogastrium with guarding. A CT scan of the abdomen and pelvis was performed (Fig. 1A).
Laparoscopic surgery was decided. During surgery, an appendiceal mass was found and appendectomy was performed.
The pathology report of the surgical specimen informed acute purulent appendicitis with periappendicitis and mesoappendicitis associated with mucinous neoplasm, low-grade dysplasia and irregular pools of mucinous material in the appendiceal wall (Fig. 1.B). Positive margin with foci of mucin within the wall.
On postoperative day 30, a new CT scan of the abdomen and laboratory tests were performed on an outpatient basis, with normal results. The patient underwent video-assisted colonoscopy. There were no lesions in the fundus of the cecum (Fig. 1.C). A biopsy sample obtained from the ostium of the appendix showed normal mucosa.
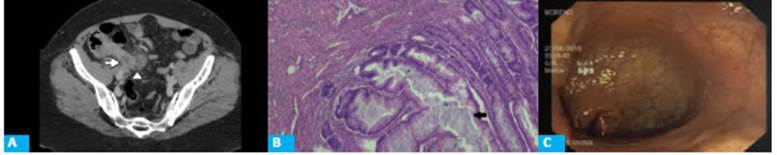
Figure 1 A: CT scan with intravenous contrast in the portal venous phase, axial section, showing vermiform appendix with a maximal diameter of 12 mm and cystic dilation within the appendiceal lumen (arrow). A collection with well- defined walls associated with changes in the surrounding fat planes (arrowhead) is observed in the tip of the appendix. B: Microscopic examination of the surgical specimen; hematoxylin and eosin stain (HE X10) shows low-grade appendiceal mucinous neoplasm associated with inflammation of the wall. A pushing like image is observed, without infiltration (arrow). C: Video-assisted colonoscopy. The fundus of the cecum is normal.
Case #2
A 39-year-old male patient without significant history asked for medical advice due to abdominal pain which had started 9 days before in the epigastric region and then migrated to the right iliac fossa, without accompanying symptoms. A CT scan of the abdomen and pelvis was performed (Fig. 2.A).
The patient underwent laparoscopic surgery. The vermiform appendix was enlarged, and a tumor was visualized, The appendix was resected.
The pathology report informed the presence of a distal cyst in the vermiform appendix, LNMA and clear margins (Fig. 2.B).
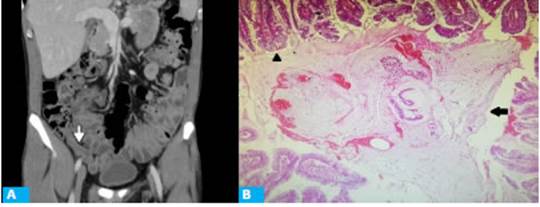
Figure 2 A: CT scan with intravenous contrast in the portal venous phase, coronal view showing vermiform appendix with a maximal diameter of 10 mm (arrow). The surrounding fat planes are preserved. B: Microscopic examination of the surgical specimen; hematoxylin and eosin stain (HE x4) shows luminal dilation and intraluminal accumulation of mucus occupied by mucinous material (arrow) and lining of the mucosa by low grade villous type epithelium (arrowhead).
Case #3
A male patient aged 47 years without significant past history complaint of diffuse abdominal pain that had started 72 hours before and migrated to the right iliac fossa within the past 12 hours, accompanied by decreased appetite and fever. A CT scan of the abdomen and pelvis was performed (Fig. 3.A).

Figure 3 A: CT scan with intravenous contrast in the portal venous phase, axial view, showing vermiform appendix with a maximal diameter of 11 mm (arrow), inflammatory changes in the surrounding fat and thickening of the distal ileum walls (arrowhead). B. Histology of surgical specimen (hematoxylin-eosin, magnification x4), showing replacement of normal epithelium by mucinous epithelium with signs of low grade atypia (arrow). No underlying lymphoid tissue is observed.
Case #4
A 77-year-old male patient with a history of benign prostatic hyperplasia and hypertension was admitted with lower abdominal pain and fever. The pain had started in the periumbilical region 24 hours before. A CT scan of the abdomen and pelvis was performed (Fig. 4.A). During laparoscocopy, appendicular peritonitis and an appendiceal mass were found. The pathology report informed LAMN associated with acute appendicitis, with mucinous lakes in the muscular layer in the distal end (Fig. 4.B).
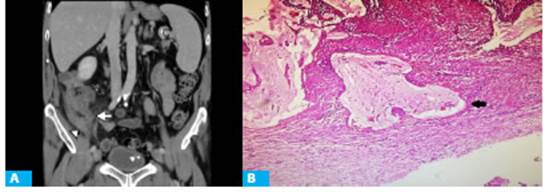
Figure 4 A: CT scan with intravenous contrast in the portal venous phase, coronal view, showing vermiform appendix with a maximal diameter of 11 mm (arrow) associated with changes in the surrounding fat and thickening of the distal ileum walls (arrowhead). B. Histology of surgical specimen (hematoxylin-eosin, magnification x4) showing mucinous lakes in the wall of the appendix (arrow). The patient underwent video-assisted laparoscopy during which appendicular peritonitis was observed with an appendiceal mass in the right iliac fossa. The pathology report informed LAMN associated with acute purulent appendicitis (Fig. 3.B).
Discussion
In 2019, 303 appendectomies were performed in our hospital. The postoperative diagnosis of AMN was made with the pathology report in 4 of these patients.
As this condition is very rare, we considered that this number is very significant, and thus we decided to present this paper.
In our experience, all the patients presented acute symptoms suggestive of acute appendicitis. The most common sign was abdominal pain in the right iliac fossa with localized guarding, absence of signs of peritonitis, and sequence of Murphy.
The CT scan images were not suggestive of AMN and the diagnosis was always retrospective. Although the prevalence is higher in women with a female-to-male ratio of 4:1, we observed that 75% of our patients were men. This can be explained by a bias due to the low number of patients in our population, so we cannot draw a conclusion about the demographic characteristics of this disease.
Conclusion
In the presence of chronic pain in the right iliac fossa with acute abdomen due to an acute appendicitis with a luminal diameter > 13 mm or wall calcifications in imaging tests, the diagnosis of AMN should be suspected2,3.
In these cases, extreme care must be taken during surgery to avoid perforation of the appendicular wall and the subsequent dissemination of neoplastic cells or mucus into the peritoneum13,16.
One of the most dreadful complication of AMN is PMP; thus, postoperative follow-up is crucial in these patients2.
Referencias bibliográficas /References
1. Yanagawa S, Yoshinaka H, Tanji H, Kodama S, Takeshima Y, Sumimoto K. Rare cases of low-grade appendiceal mucinous neoplasm: Two case reports and a literature review. Case Rep Oncol. 2019[citado 10/03/2020]; 12:488-93. Disponible en Disponible en https://doi.org/10.1159/000501307 [ Links ]
2. Saylam B, Güldogan C, Coskun F, Vural V, Comcali B, Tez M. Distinguishing between acute appendicitis and appendiceal mucocele: is this possible preoperatively?. Eur J Trauma Emerg Surg. 2013[citado 10/03/2020]; 5:523-9. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/26815451 [ Links ]
3. Bennett GL, Tanpitukpongse TP, Macari M, Cho KC, Babb JS. CT diagnosis of mucocele of the appendix in patients with acute appendicitis. AJR Am J Roentgenol. 2009[citado 10/03/2020]; 192:103-10. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/19234237 [ Links ]
4. Glasglow SC, Gaertner W, Stewart D, Davids J, Alavi K, Paquette IM, et al. The American Society of Colon and Rectal Surgeons, Clinical Practice Guidelines for the Management of Appendiceal Neoplasms. Dis Colon Rectum. 2019[citado 10/03/2020]; 62:1425-38. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/31725580 [ Links ]
5. Wong M, Barrows B, Gangi A, Kim S, Mertens RB, Dhall D. Low- Grade Appendiceal Mucinous Neoplasms: A Single Institution Experience of 64 Cases With Clinical Follow-up and Correlation with the Current (Eighth Edition) AJCC Staging. Int J Surg Pathol. 2020[citado 30/07/2020]; 3:252-8. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/31645160 [ Links ]
6. Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, et al. The histopathological classification, diagnosis and differential diagnosis of mucinous appediceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. 2017[citado 10/03/2020];71:847-858. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/28746986 [ Links ]
7. Carr NJ, Cecil TD, Mohamed F, Sobin LH, Sugarbaker PH, González Moreno S, et al. A Consensus for Classification and Pathologic Reporting of Pseudomyxoma Peritonei and Associated Appendiceal Neoplasia: The Results of the Peritoneal Surface Oncology Group International (PSOGI) Modified Delphi Process. Am J Surg Pathol. 2016[citado 10/03/2020]; 40:14-26. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/26492181 [ Links ]
8. Odze R, Goldblum J. Patología quirúrgica del tracto gastrointestinal. 3a ed. Filadelfia: Amolca; 2017. [ Links ]
9. Valasek MA, Pai RK. An update on the diagnosis, grading and staging of appendiceal mucinous neoplasms. Adv Anat Pathol. 2018[citado 10/03/2020]; 25:38-60. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/29016471 [ Links ]
10. World Health Organization. Digestive system tumours . 5th ed. Lyon, France: WHO Classification of Tumours; 2018. [ Links ]
11. Pai RK, Hartman DJ, Gonzalo DH, Lai KK, Downs Kelly E, Goldblum JR, et al. Serrated lesions of the appendix frequently harbor KRAS mutations and not BRAF mutations indicating a distinctly different serrated neoplastic pathway in the appendix. Hum Pathol. 2014[citado 11/03/2020]; 45:227-35. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/24439221 [ Links ]
12. Khan MR, Ahmed R, Saleem T. Intricacies in the surgical management of appendiceal mucinous cystadenoma: a case report and review of the literature. J Med Case Rep. 2010[citado 11/03/2020]; 4:129. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/20444269 [ Links ]
13. Carr N, Arends M, Deans G, Sobin L (editors). Tumours of the appendix, adenocarcinoma of the appendix. En: Hamilton Stanley R, Aaltonen Lauri A. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press; 2000. pp. 93-8. [ Links ]
14. Arnason T, Kamionek M, Yang M, Yantiss RK, Misdraji J. Significance of proximal margin involvement in low-grade appendiceal mucinous neoplasms. Arch Pathol Lab Med. 2015[citado 11/03/2020]; 139:518-21. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/24971927 [ Links ]
15. Sugarbaker PH. Epithelial appendiceal neoplasms. Cancer J. 2009[citado 11/03/2020]; 15:225-35. Disponible en Disponible en https://www.ncbi.nlm.nih.gov/pubmed/19556909 [ Links ]
16. Rouchaud A, Glas L, Gayet M, Bellin M. Appendiceal mucinous cystoadenoma. Diagn Interv Imag. 2014[citado 11/03/2020]; 95:113-6. Disponible en Disponible en https://pdfs.semanticscholar.org/efbe/34cb16bffeab740be107b3d69b5f8ed9e10b.pdf [ Links ]
17. Carmignani PC, Hampton R, Sugarbaker CE, Chang D, Sugarbaker PH. Utility of CEA and CA 19-9 Tumor Markers in Diagnosis and Prognostic Assessment of Mucinous Epithelial Cancers of the Appendix. J Surg Oncol. 2004[citado 11/03/2020]; 87:162-6. Disponible en Disponible en https://pubmed.ncbi.nlm.nih.gov/15334630/ [ Links ]
18. Honoré C, Caruso F, Dartigues P, Benhaim L, Chirica M, Goéré D, et al. Strategies for Preventing Pseudomyxoma Peritonei After Resection of a Mucinous Neoplasm of the Appendix. Anticancer Res. 2015[citado 11/03/2020]; 35:4943-7. Disponible en Disponible en http://ar.iiarjournals.org/content/35/9/4943.long [ Links ]
19. Younes M, Katikaneni PR, Lechago J. Association between mucosal hyperplasia of the appendix and adenocarcinoma of the colon. Histopathology. 1995[citado 11/03/2020 ]; 26:33-7. Disponible en Disponible en https://pubmed.ncbi.nlm.nih.gov/7713482/ [ Links ]
Received: May 12, 2020; Accepted: September 09, 2020











 texto em
texto em