Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.113 no.2 Cap. Fed. jun. 2021
http://dx.doi.org/10.25132/raac.v113.n2.1496.ei
Articles
Endoscopic treatment of intramucosal adenocarcinoma in hyperplastic gastric polyp
1 Servicio de Cirugía General. Sanatorio Allende. Córdoba, Argentina.
2 Servicio de Gastroenterología y Endoscopia Terapéutica. Sanatorio Allende. Córdoba, Argentina.
The prevalence of gastric polyps (GPs) during upper gastrointestinal endoscopies is 6%1-3, and 17% correspond to gastric hyperplastic polyps (GHPs) 3. They occur with equal incidence in men and women3, are usually asymptomatic and found incidentally during endoscopic examinations2,3.
However, when their size increases, they can cause symptoms such as anemia, gastrointestinal bleeding, and gastric outlet obstruction, and progress to adenocarcinoma3.
Some studies report a prevalence of metaplasia of 5.6% in GHPs, while dysplasia and gastric cancer (GC) correspond to 3.3% and 2.1%, respectively1-3.
The aim of this paper is to describe an atypical presentation of this disease with review of the literature.
A 73-year-old male patient visited the emergency department due to progressive dyspnea and palpitations within the past month. He was a former smoker and had a history of hypertension, trifascicular block and atrial fibrillation requiring pacemaker, type 2 diabetes mellitus, hypothyroidism, overweight, chronic kidney disease and right radical nephrectomy due to renal oncocytoma. The physical examination was normal. The abnormal findings of the laboratory tests were hemoglobin 7.95 mg/dL, hematocrit 27% INR 1.23 and prothrombin time 62%. A diagnosis of hypochromic anemia due to iron deficiency was made and the patient was admitted to the coronary care unit for evaluation.
The upper gastrointestinal endoscopy showed a pedunculated polyp, 3 mm in size in the gastric angle; ulceration was not present. The pathology report described severe dysplasia with carcinoma in situ without invasion of the lamina propria. The evaluation was completed with a colonoscopy, which reported diverticula without complications, and internal hemorrhoids.
The patient underwent endoscopic polypectomy with endoloop, and the specimen was resected in bloc (Fig. 1).
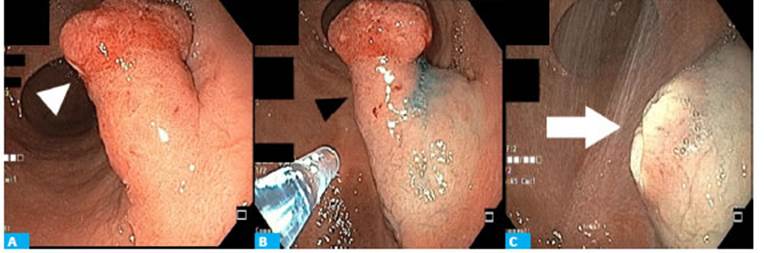
Figure 1 Endoscopic resection with endoloop. A. 3-mm gastric polyp (white arrowhead). B. Injection of saline solution before resection (black arrowhead). C. Surgical bed without signs of bleeding (white arrow).
The pathology report revealed the presence of hyperplasia and moderately differentiated intramucosal adenocarcinoma (pT1a) without ulceration; the stalk was free of lesion. The lesion was completely resected with a margin > 2 mm and absence of lymphovascular infiltration (Fig. 2).
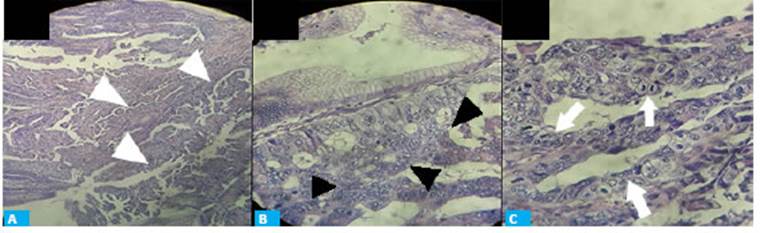
Figura 2 Histological examination. A. Histological changes suggestive of adenocarcinoma, abnormalities of stromal structure (white arrowheads), hematoxylin and eosin staining x20. B. Hyperplastic stromal glands. Nuclear atypia (black arrowheads), hematoxylin and eosin staining x40. C. Cells in mitosis, nuclear anisokaryosis (white arrows), hematoxylin-eosin staining ×100
The polyp with adenocarcinoma foci was classified as early gastric cancer (EGC) pT1a of the TNM staging system4,5. Therefore, endoscopic resection with endoloop fulfilled the criteria of curative resection4-6. The histopathology of the surrounding mucosa revealed moderate chronic gastritis in the body and antrum of the stomach without evidence of Helicobacter pylori.
The patient evolved with favorable outcome and was controlled three months later with an upper gastrointestinal endoscopy with negative biopsies and was included in the protocol of endoscopic surveillance.
The risk for cancer in GHP is higher when the polyp size increases2, particularly in polyps > 2 cm1; yet, GC has been described in polyps > 5-10 mm1. Other risk factors are pedunculated polyps, remnant stomach after gastrectomy, and synchronous dysplasia3.
Nowadays, resection is indicated in symptomatic GHPs, those > 2 cm2,3, with focal dysplasia and in EGC1-3.
Once the presence of dysplasia is confirmed, its grade and severity should be determined, and also if it is limited to the polyp or is a fragment of the neoplasm1.
The depth of penetration of EGC in a GHP can be assess only when the cross-sectional image perpendicular to the lesion and contiguous normal wall are obtained1.
The risk of developing gastric cancer in the gastric mucosa surrounding the GHPs is 7.1%; therefore, the adjacent regions of the gastric mucosa must also undergo endoscopic and anatomopathological evaluation, and assessment of the presence of Helicobacter pylori1.
Several protocols have been developed to improve the early diagnosis of GC, which suggest the detection and eradication of Helicobacter pylori in patients undergoing upper gastrointestinal endoscopy, the diagnosis of gastric atrophy or gastric metaplasia in all symptomatic patients > 40 years, and the identification of focal lesions through the use of high-definition white light endoscopy6.
Endoscopic treatment of GHP with dysplasia or cancer is considered sufficient if it has been completely resected according to the endoscopist (macroscopic radicality) and histopathologist (microscopic radicality)1,2.
When the polyp is removed with a diathermic loop it is less probable that some advanced dysplastic and neoplastic lesions can be missed and more likely that total removal is accomplished1.
If the cancer does not exceed the gastric mucosa, the excision margin free of cancer cells is > 2 mm in the microscopic investigation, differentiation degree of the cancer is high or moderate and no angioinvasion is observed, the resection is considered oncologically radical1,2,4-6.
Therefore, endoscopic resection with endoloop fulfilled the criteria of curative resection1,4-6.
The percentage of cancer relapse after radical resection of GHPs containing focal cancer is unknown, although it is lower than that after the endoscopic resection of nonpolypoid EGC (1.2%)1.
Oncological surveillance of patients with GHPs containing foci of dysplasia and cancer should be patient-tailored, since there are no generally accepted guidelines1. Yet, some studies suggest that the oncological surveillance should include upper gastrointestinal endoscopy, first one year after and then 3 years after the initial polypectomy2.
An experienced endoscopist should perform surveillance using high-definition white light endoscopy. Chromoscopy can improve the characterization of the lesions, guide biopsies and define the edges of an eventual resection6.
In conclusion, we have reported a case of an EGC in a GHP, constituting a diagnostic and therapeutic challenge. We believe it is essential to consider minimally invasive treatment by endoscopic resection, as it is considered sufficient in this type of patients1,2,4-6.
Referencias bibliográficas /References
1. Markowski AR, Markowska A, Guzinska-Ustymowicz K. Pathophy siological and clinical aspects of gastric hyperplastic polyps. World J Gastroenterol. 2016; 22(40):8883-91. doi:10.3748/wjg.v22.i40.8883 [ Links ]
2. Markowski AR, Guzinska-Ustymowicz K. Gastric hyperplastic polyp with focal cancer. Gastroenterol Rep. 2016; 4(2):158-61. doi:10.1093/gastro/gou077 [ Links ]
3. Park KS, Lee SW, Lee J, et al. Small gastric hyperplastic po lyp with acute bleeding as an unusual presentation of malig nancy. Med (United States). 2018; 97(22):0-3. doi:10.1097/MD.0000000000010899 [ Links ]
4. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014. Gastric Cancer. 2017; 20(1):1-19. doi:10.1007/s10120-016-0622-4 [ Links ]
5. Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016; 28(1):3-15. doi:10.1111/den.12518 [ Links ]
6. Rollán A, Cortés P, Calvo A, et al. Recommendations of the chilean association for digestive endoscopy for the management of gas tric pre-malignant lesions. Rev Med Chil. 2014; 142(9):1181-92. doi:10.4067/S0034-98872014000900013 [ Links ]
Received: August 05, 2020; Accepted: September 23, 2020











 texto em
texto em 


