Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.115 no.4 Cap. Fed. dez. 2023 Epub 29-Nov-2023
http://dx.doi.org/10.25132/raac.v115.n4.1724
Original article
Chronic intestinal failure due to short bowel syndrome: current role of surgery, medical rehabilitation, and intestinal transplantation. A progress report after 16 years of multidisciplinary management
1Unidad de Soporte Nutricional, Rehabilitación y Trasplante Intestinal. Servicio de Cirugía General, Trasplante Hepático, Pancreático e Intestinal. Hospital Universitario. Fundación Favaloro. Buenos Aires. Argentina
Background:
Severe intestinal insufficiency is known as chronic intestinal failure (CIF). In recent decades, medical treatments and surgical procedures have been incorporated, developed and improved under the name intestinal rehabilitation. When performed by multiand interdisciplinary teams, these treatments have high success rates.
Objective:
The aim of present study is to describe the 16-year outcomes in the management of patients with CIF secondary to short bowel syndrome (SBS) and the role of surgery, medical rehabilitation, and transplantation.
Material and methods:
We conducted a retrospective analysis on a prospective database of patients treated with chronic intestinal failure due to SBS between February 2006 and March 2022.
Results:
A total of 492 patients (368 adults (Group A) and 124 pediatric patients (Group B)] were included. Group A: 111 patients underwent autologous gastrointestinal reconstruction surgery (AGIRS), 16 were treated with semisynthetic glucagon-like peptide-2 (sGLP2); 83% achieved rehabilitation, with 77% survival at 10 years; 6.8% (17 patients) required intestinal transplantation (ITx), with 89% independence from parenteral nutrition (PN) at 1 year and post-ITx survival of 29% at 10 years. Group B: 18 patients underwent AGIRS; 9 underwent serial transverse enteroplasty (STEP); and 6 received sGLP2; 52% were rehabilitated, with 69% survival at 10 years; 28 patients received ITx, with 69% independence from PN at 1 year and 39% survival at 10 years.
Conclusion:
These results highlight the central role of surgery and medical rehabilitation in the recovery of intestinal function.
Keywords: Short Bowel Syndrome; Intestinal Failure; Parenteral Nutrition; Rehabilitation; Intestinal Transplant
Introduction
Parenteral nutrition (PN) created a paradigm shift in the understanding and management of intestinal failure (IF). It has become the main means of support for acute patients, while home parenteral nutrition (HPN) remains the primary life-saving treatment for patients with chronic intestinal failure1. The development of intestinal transplantation (ITx) provided an alternative to restore intestinal function2,3,4 and became a consolidated therapy with the introduction of calcineurin inhibitors, first with cyclosporine and with tacrolimus thereafter5,6.
The need to perform surgery on patients with short bowel syndrome (SBS) without indication for ITx has resulted in various procedures for intestinal rehabilitation surgery (IRS).
Intestinal lengthening techniques, as the Longitudinal Intestinal Lengthening and Tailoring (LILT) procedure developed by Binachi7 and the Serial Transverse Enteroplasty (STEP) described by Kim in 20038, contributed to improve the intestinal rehabilitation rate. Autologous gastrointestinal reconstruction surgery (AGIRS) brought together procedures that not only restore intestinal continuity but also improve the initially unfavorable anatomy for one more favorable, resulting in a prognostic marker for adults and children9,10.
Messing and Amiot, in 1999 and 2013, respectively, determined that PN dependence in adult patients with chronic intestinal failure (CIF) due to SBS depended on postoperative anatomic factors, such as post-surgical intestinal length (< 75 cm), absence of ileocecal valve (ICV), colon in continuity < 57%, and plasma citrulline concentration < 20 µmol/L11,12.
The inclusion of the semisynthetic glucagon-like peptide-2 analogue (sGLP2) in 2012 for the treatment of adult patients with IF due to SBS and benign conditions changed the course of IF, enabling those with unfavorable postoperative anatomies to achieve enteral autonomy13,14,15. In 2015, the European Society for Clinical Nutrition and Metabolism (ESPEN) revised the definition of intestinal failure (IF), differentiating it from intestinal insufficiency. In addition, the guideline committee outlined a functional, a pathophysiological, and a clinical classification, and identified three anatomic types in SBS16. Intestinal failure has been recognized as the most recent form of organic failure, categorized as an orphan disease. Due to its complex nature, it must be managed by specialized multidisciplinary and interdisciplinary teams17,18,19. In 2006, we established the first multidisciplinary team in Argentina, specialized in the management of these patients.
The aim of present study was to describe the 16-year outcomes in the management of patients with CIF secondary to SBS and the role of surgery, medical rehabilitation, and ITx over the years.
Material and methods
We conducted a retrospective analysis on a prospective database consisting of adult and pediatric patients treated for CIF due to SBS between February 2006 and March 2022. Patients who underwent IRS (AGIRS or STEP) or ITx were included in the analysis. We excluded patients who underwent medical rehabilitation without surgery or those who received intestinal reconstruction at other centers.
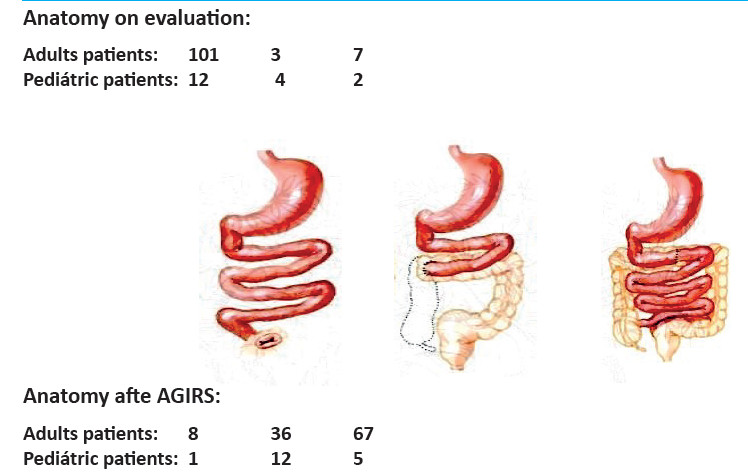
The variables analyzed in candidates for IRS at the first consultation were: age, sex, height, weight (pediatric Z-score), subjective global assessment (SGA) in adults (A: well nourished; B: moderately malnourished; C: severely malnourished)20, causes of SBS, type of anatomy [type 1: end-jejunostomy; type 2: jejunocolonic anastomosis; type 3: jejunoileal anastomosis with preservation of the ileocecal valve (ICV]) and a part of or the entire colon in continuity], and time in HPN.
After IRS, the following variables were analyzed: surgery performed, type of anatomy and post-surgical intestinal length (PSIL), [considering in adults: A ≤ 50 cm, B: 51-99 cm, C: 100 cm; and in children: A: ≤ 40 cm, B: 41-79 cm and C: ≥ 80 cm9 (intestinal length measured in the operating room, as published by Gondolesi et al.)21], post-surgical medical treatment, percentage of patients and time to discontinuation of HPN, overall survival and percentage of patients who required ITx. IN the ITx group we analyzed the indications and type of ITx performed, time to discontinuation of HPN, and patient and graft long-term survival.
Post-surgical medical treatment was divided into two periods. Initially, conventional medication was administered, and included antimotility drugs, antisecretory drugs, antibiotics, pancreatic enzymes and bile acid sequestrants. In the second period, an sGLP2 was added in 2014 for adults and in 2016 for pediatric patients. Patients who met the specific inclusion and exclusion criteria outlined in our protocol were eligible for this treatment22.
The results were analyzed by age group (adult/ pediatric patients) and the period of treatment.
All the calculations were made using Social Package for Social Sciences (SPSS) version 20.0. A p value or log rank test < 0.05 was considered statistically significant. The study was approved by the institutional review board (DDI [1477] 1119).
Results
A total of 492 patients with IF were evaluated; Sub-analysis by periods (Fig. 4): At the first visit, most patients were malnourished in both periods according to the SGA: 77.8% in the first period (VGS 368 were adult patients and 124 were pediatric patients (Fig. 1).
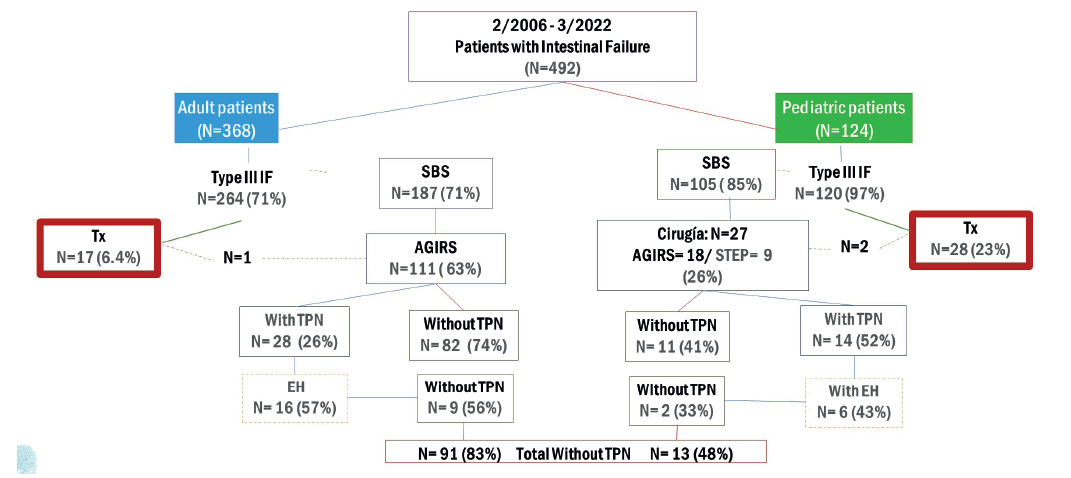
FIGURE 1 Flow chart. Seventeen adult patients received 18 transplants, and 28 pediatric patients received 33 transplants. TPN: total parenteral nutrition; EH: enterohormones; SBS: short bowel syndrome; AGIRS: autologous gastrointestinal rehabilitation surgery; Tx: transplantation.
Adult patients
Surgical rehabilitation
Of the 368 patients with IF, 264 (72%) had CIF and was due to SBS in 187 (71%) (Fig. 1).
The most frequent etiologies were postoperative complications (55%), bowel ischemia (21%), trauma (5.4%) and complications of bariatric surgery (5.4%).
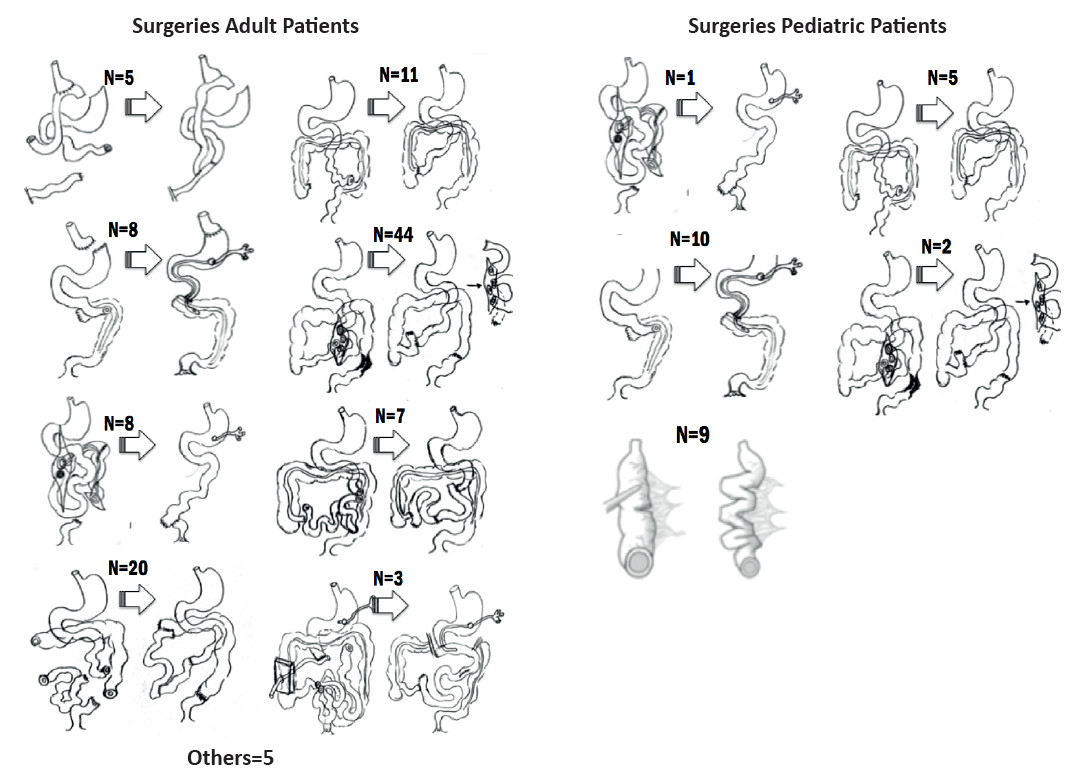
Autologous gastrointestinal reconstruction was performed in 111/187 patients with CIF (Fig. 2). Mean age was 49 ± 15 years and 61 (55%) were men. Five patients required two procedures and one patient required three procedures to restore intestinal continuity. In the first visit. 101 patients (90.1%) had type 1 anatomy, 7 patients (6.3%) had type 3 anatomy, and 3 patients (2.7%) had type 2 anatomy. After AGIRS, 67 patients (60.4%) were converted to type 3 anatomy, 36 (32.4%) were left with type 2 anatomy, and only 8 (7.2%) remained with type 1 anatomy (p < 0.00001) (Fig. 3).
Mean postoperative intestinal length was 155.3 ± 102.6 cm: “A” (20 ± 14 cm) in 23 patients, “B” (76.6 ± 13 cm) in 21 patients and “C” (208.4 ± 75 cm) in 67 patients. Time on HPN before surgery was 350.6 ± 296.4 days. With the standard medical treatment after surgery, 82/111 patients (74%) discontinued HPN after 268.9 ± 416.3 days (Fig. 1).
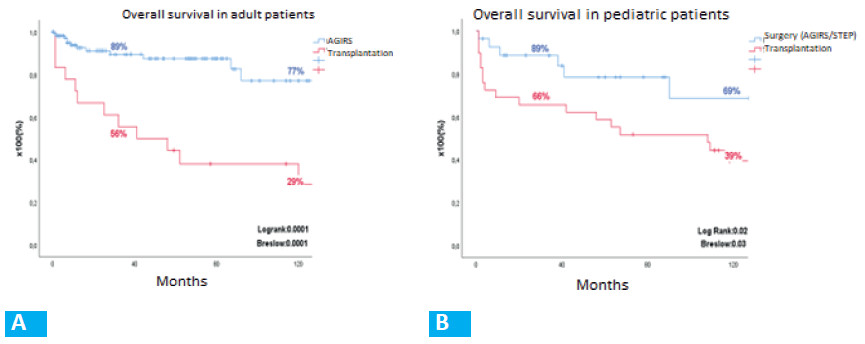
Sub-analysis by periods (Fig. 4): At the first visit, most patients were malnourished in both periods according to the SGA: 77.8% in the first period (VGS “B”: 42.2%; “C”: 35.6%) and 78.8% in the second one (VGS “B”: 37.9%; “C”: 40.9%). Medical nutrition therapy improved this condition to “A” in 74.3% and 60.6% in each period. Autologous gastrointestinal reconstruction was performed on 45 patients in the first period and on 66 patients in the second. In the first period, PN was discontinued in 32/45 patients (71.1%) within 409.5 ± 644.3 days, with a SGA category “A” in 30 (93.8%) and “B” in 2 (6.2%). In the second period, PN was discontinued in 39/66 patients (59.1%) within 128.3 ± 188.3 days. The nutrition status was A in 36 patients (92.3%) and “B” in 3 (7.7%) (Fig. 5). Overall survival was 89 at 3 years and 77% at 10 years (Fig. 6). Forty patients continued with HPN, 13 in the first period and 27 in the second period (Figs. 4B y 5B). Sixteen patients (5 from the first period and 11 from the second) met the criteria of our protocol to receive sGLP2. Nine patients (56.3%) discontinued HPN within 369.1 ± 363.8 days, with an average PSIL of 57.2 ± 46.1 cm. The remaining 7 patients responded to treatment and decreased the initial HPN volume by more than 20% or discontinued HNP 2 days a week.
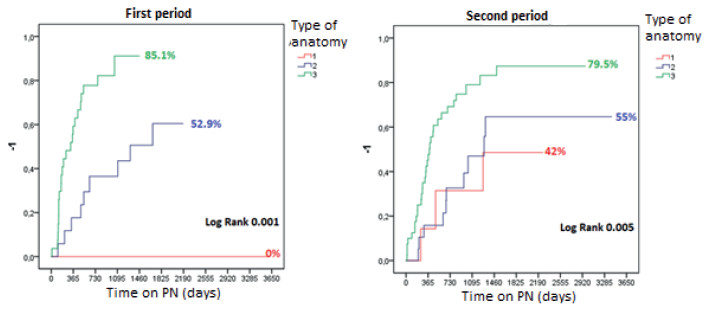
FIGURE 4 Time to achieve independence from PN after AGIRS, according to the type of postoperative anatomy and the period evaluated in adult patients.
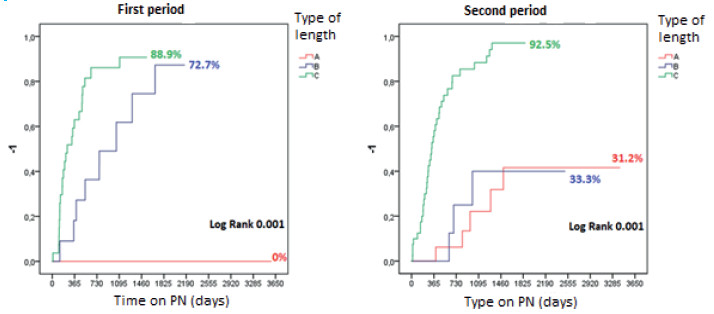
FIGURE 5 Time to achieve independence from PN after AGIRS, according to post-surgical in-testinal length and the period evaluated in adult patients.
Intestinal transplantation
A total of 18 ITx procedures were performed on 17 patients: 12 were isolated transplantations, 3 were multi-visceral transplantations (1 in combination with a kidney transplant), 2 were liver transplants (1 in combination with a kidney transplant), and 1 was a modified multi-visceral transplantation. The indications included loss of venous access due to thrombosis in 9 patients (50%); liver disease associated with IF in 4 (22.2%); recurrent catheter infections in 2 (11%); diffuse portomesenteric thrombosis in 1 patient, and other indications in 2 patients. Fourteen ITx were performed during the first period and 4 during the second period. Mean time on HPN before ITx was 1760 ± 2064 days. Independence from HPN was reached on day 70 ± 56 after ITx (p = 0.0001). Survival at 3 and 10 years after ITx was 56 and 29%, respectively. Sixteen years after ITx, 6 patients are alive, 5 of them with functional graft and 1 on the waiting list for re-transplantation.
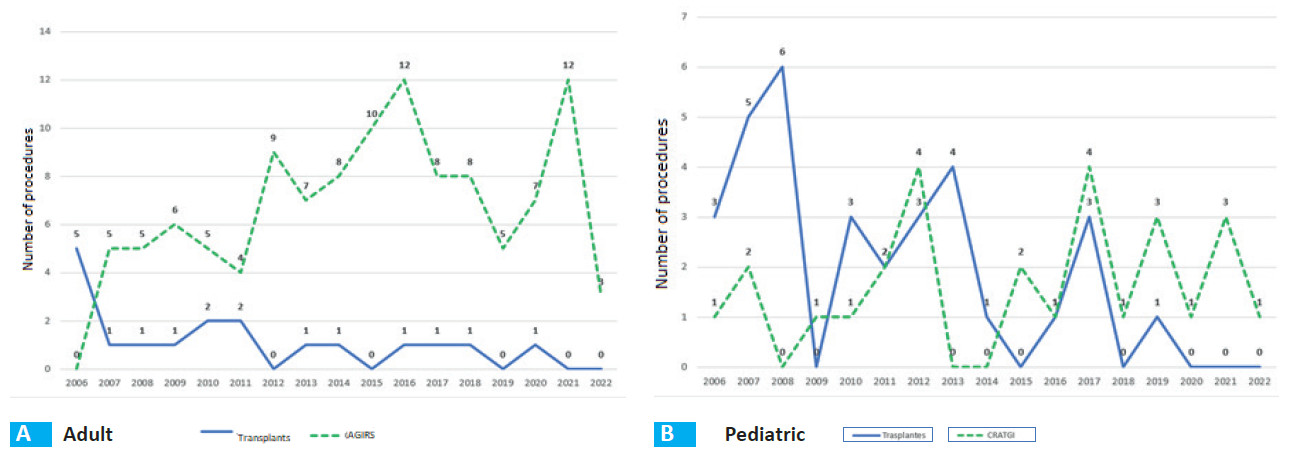
Long-term survival after AGIRS vs. ITx is significantly different (log-rank test = 0.0001) (Fig. 6A). Figure 7A shows the variability of the indication for surgical procedures (AGIRS) and ITx over time by year.
Pediatric patients
Surgical rehabilitation
Of the 124 patients with IF, 120 (96.8%) had CIF and 105 of them were due to SBS (87.5%) (Fig. 1). The most frequent etiologies were intestinal atresia (26%), gastroschisis (18.5%), volvulus (18.5%) and necrotizing enteritis (11%). A total of 27/105 patients underwent IRS, 18 AGIRS and 9 STEP. Mean age was 8 ± 5 years, 17 (63%) were male, height was 84 ± 32 cm and weight was 12.6 ± 11.5 kg (height-for-age Z score: -2.55 ± 1.9; weight-for-age Z score: -2.67 ± 2.02). The surgical procedures performed are shown in Figure 2. Among patients eligible for AGIRS in the first visit, 12 patients (66.7%) had type 1 anatomy, 4 patients (22.2%) had type 2 anatomy, and 2 patients (11.1%) had type 3 anatomy. After surgery, 12 patients (66.7%) were converted to type 2 anatomy, 5 (27.7%) were converted type 3 anatomy, and 1 (5.5%) remained with type 1 anatomy (p < 0.0003). Among patients eligible for STEP, 4 patients had type 2 anatomy and 5 patients had type 3 anatomy. Mean PSIL was 31 ± 18 cm: “A” (21 ± 10 cm) in 10 patients, “B” (58 ± 12 cm) in 9 patients and “C” (111 ± 30 cm) in 8 patients. Time on HPN before surgery was 684 ± 878 days and 11/27 patients (41%) discontinued HPN within 860 ± 1130 after surgery (Fig. 8 and 9).
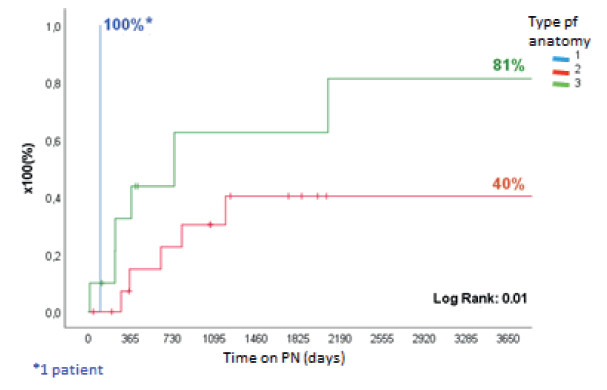
FIGURE 8. Time to achieve independence from PN after AGIRS + STEP, according to the type of postoperative anatomy in pediatric patients.
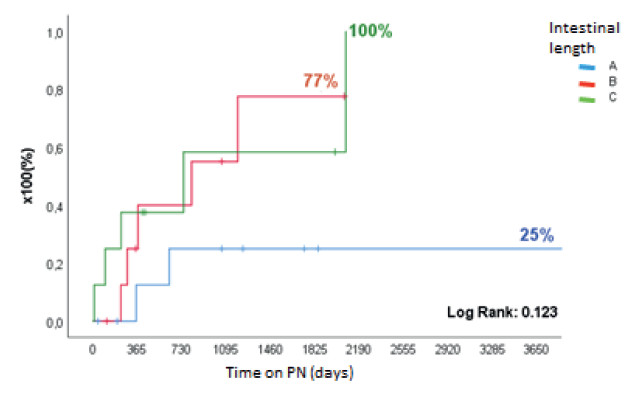
FIGURE 9. Time to achieve independence from PN after AGIRS + STEP, according to post-surgical intestinal length in pediatric patients.
Sub-analysis by periods: Of the 27 IRS performed, 13 (8 AGIRS and 5 STEP) took place in the first period and 14 (10 AGIRS and 4 STEP) in the second period. In the first period, 9/13 patients (69%) discontinued HPN within 540 ± 328 days and, in the second period, 2/14 patients (14.3%) discontinued HPN within 58 ± 64 days with standard medical postoperative treatment. The length of the follow-up period for this group is insufficient to compare it with the first group. In the second period, 6 patients received treatment with sGLP2. Post-surgical intestinal length was 40 ± 10 cm, 5 patients had type 2 anatomy and 1 patient had type 3 anatomy. Home parenteral nutrition was discontinued in 2/6 patients (33%) on day 275 and 204, respectively. The remaining 4 patients (66%) responded to treatment and decreased the initial HNP volume by 20% or discontinued HNP 2 days a week. Overall survival after surgery was 89% at 3 years and 69% at 10 years (fig. 6B). In the last visit, height was 113 ± 32 cm and weight was 22 ± 13 kg (height-for-age Z score: -2.21 ± 1.8, and weight-for-age Z score -2.56 ± 2.1).
Intestinal transplantation
A total of 33 ITx were performed: 28 were primary transplants, 4 were re-transplantation procedures and 1 patient underwent a second re-transplantation. The primary ITx included 24 isolated transplantations, 3 were combined with liver transplantations, and 1 multivisceral transplantation. Among retransplantations, 3 were isolated procedures, 1 was in combination with liver and kidney transplantation and 1 was multivisceral in combination with kidney transplantation.
The indications included loss of venous access in 15 patients (52%); liver disease associated with IF in 6 (20.7%); recurrent catheter infections in 3 (10%); and other indications in 4 patients.
Mean length of the intestine implanted was 325 ± 59.4 cm. Mean time on HPN before ITx was 1491 ± 1642 days, and HPN was discontinued within 66 ± 57 days after ITx (p = 0.0001). By the end of the first year, 69% remained independent of HPN. Actuarial survival at 3 and 10 years was 66% and 39%, respectively (logrank test: 0.02) (Fig. 6B). By the end of the observation, 14 patients (48%) are alive, 9 (31%) of them with functional graft and 5 on HPN.
Figure 7B shows the variability of the indication for surgical procedures (AGIRS) and ITx over time by year for this group.
Discussion
Argentina was the first country in Latin America to implement hospital and home PN for adult and pediatric patients23. In 2006, Hospital Universitario Fundación Favaloro created the first multidisciplinary unit for nutritional support, intestinal rehabilitation, and intestinal transplantation in both Latin America and our country. This entailed providing training to all relevant areas, developing a clinical and surgical work modality, implementing prospective management protocols, and integrating international therapies. The first patients referred to this unit were those who had already developed complications associated with prolonged HPN, with indication for ITx. Thus, the highest number of ITx procedures occurred between 2006 and 2008, enabling the reporting of the initial successful series in the region24. Implementing IRS has consolidated AGIRS and STEP as surgical tools to improve the initial anatomy and increase the chances of rehabilitation, as Abu Elgmad et al.25 reported on their experience with 500 adult patients with CIF. Similar results were achieved by Gondolesi et al. after analyzing the management of IF in emerging countries26. However, the introduction of sGLP2 in intestinal rehabilitation has significantly changed the course of this disease for patients with unfavorable post-surgical anatomies. This therapy has allowed these individuals to attain recovery of intestinal function. In 2019, our unit published its initial experience and the first formula to predict the outcome following AGIRS among 88 adult patients undergoing medical rehabilitation based on a protocol that included the use of sGLP2, resulting in discontinuation of PN in 83% of patients27. Our protocol for postsurgical rehabilitation with the use of sGLP2, published in 2020, determines administration of standard medical-nutritional treatment to all patients after IRS. Treatment with sGLP2 should only be considered for those whose NP volume cannot be decreased after 6 months22. With this protocol, only 14.4% of our patients were eligible to receive sGLP2, in contrast to 48 and 35.4% reported by other centers28,29. In addition, we achieved a high rate of intestinal rehabilitation with few adverse effects, even in patients with very unfavorable anatomy (PSIL < 10 cm, absence of IVC and partial colon in continuity). These results were included in the first meta-analysis on the subject published in 202230.
For pediatric patients, our unit exclusively receives complex referrals, with 97% of patients referred for CIF. As in adults, the first pediatric patients referred had complications from prolonged HPN and indication for ITx. Unfortunately, the donor shortage affected waiting list mortality31, leading to a rise in IRS that exceeded the indication of ITx. The PSIL was 31 cm, reflecting disease severity in this age group. With the standard medical treatment after surgery, 44% of the patients discontinued PN, and with the use of sGLP2, intestinal rehabilitation increased to 53%. As reported by the Necker Hospital, of 36 patients with small bowel length A, 36% discontinued PN and 12 required ITx32. In a series of 139 patients, Belza et al.33 found that 68% achieved nutritional autonomy. Factors that favorably impacted this outcome included an intestinal length > 100 cm, the presence of ICV, less hepatic impact, and fewer sepsis episodes. The international results with the use of sGLP2 were similar to ours34,35. A recent systematic review on pediatric patient36 included two publications from our center37,38. Patients with ITx discontinued PN in less time than that required following IRS, because an organ with normal length and functionality is implanted in ITx39,40. Graft rejection, graft loss, and mortality from infections or diseases like post-transplant lymphoma or graft-versus-host disease can significantly affect both the functional capacity of the graft and patients’ long-term survival of the patient6,38,39.
The development of IF units in our country, coupled with the ongoing reporting of their progress, has positioned Argentina as a leader in the field both regionally and globally18,41.
Referencias bibliográficas /References
1. Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery. 1968;64(1):134-42. [ Links ]
2. Lillehei RC, Idezuki Y, Kelly WD, Najarian JS, Merkel FK, Goetz FC. Transplantation of the intestine and pancreas. Transplant Proc. 1969;1(1):230-8. [ Links ]
3. Ruiz JO, Lillehei RC. Intestinal transplantation. Surg Clin North Am. 1972;52(4):1075-91. [ Links ]
4. Starzl TE, Rowe MI, Todo S, Jaffe R, Tzakis A, Hoffman AL, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261(10):1449-57. [ Links ]
5. Hoffman AL, Makowka L, Banner B, Cai X, Cramer DV, Pascualone A, et al. The use of FK-506 for small intestine allotransplantation. Inhibition of acute rejection and prevention of fatal graft-versushost disease. Transplantation. 1990;49(3):483-90. [ Links ]
6. Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, et. al. Five hundred intestinal and multivisceral transplantations at a single center: major advances with new challenges. Ann Surg. 2009; 250(4):567-81. [ Links ]
7. Bianchi A. Intestinal loop lengthening. A technique for increasing small intestinal length. J Pediatr Surg. 1980;15:145-51. [ Links ]
8. Kim HB, Fauza D, Garza J, Oh JT, Nurko S, Jaksic T. Serial Transverse Enteroplasty (STEP): A novel bowel lengthning procedure. J Pediatr Surg. 2003;38:425-29. [ Links ]
9. Goulet O, Nader EA, Pigneur B, Lambe C. Short bowel syndrome as the leading cause of intestinal failure in early life: some insights into the management. Pediatr Gastroenterol Hepatol Nutr. 2019;22(4):303-29. [ Links ]
10. Gondolesi GE, Ortega M, Martinez MI, Rumbo C, Solar H. Autologous gastrointestinal reconstruction surgery for short bowel syndrome: the cornerstone for intestinal rehabilitation. Curr Opin Organ Transplant. 2022;27(2):148-53. [ Links ]
11. Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patient with the short bowel syndrome. Gastroenterology 1999;117:1043-50. [ Links ]
12. Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr. 2013;32(3):368-74. [ Links ]
13. Jeppesen P, Pertkiewicz M, Messing B, Iyer K, Seidner DL, O'keefe SJ, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology 2012;143:1473-81. [ Links ]
14. Schwartz L, O'keefe S, Fujioka K, Gabe SM, Lamprecht G, Pape UF, et al. Long-term Teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7:e142. [ Links ]
15. Seidner D, Fujioka K, Boullata J, Iyer K, Lee HM, Ziegler TR. Reduction of parenteral nutrition and hydration support and safety with long term Teduglutide treatment in patients with short bowel syndrome associated intestinal failure: STEPS-3 Study. Nutr Clinical Pract. 2018;33(4):520-7. [ Links ]
16. Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171-80. [ Links ]
17. Rhoda KM, Parekh NR, Lennon E, Shay-Downer C, Quintini C, Steiger E, et al. The multidisciplinary approach to the care of patients with intestinal failure at a tertiary care facility. Nutr Clin Pract. 2010;25(2):183-91. [ Links ]
18. Grainger J, Maeda Y, Donnelly S, Vaizey CJ. Assessment and management of patients with intestinal failure. A multidisciplinary approach. Clin Exp Gastroenterol. 2018;11:233-41. [ Links ]
19. Jung SM, Lee S, Park HJ, Kim HJ, Min JK, Seo JM. Multidisciplinary intestinal rehabilitation in acute type II intestinal failure. Results from an intestinal rehabilitation team. Asian J Surg. 2021;44(3):549-52. [ Links ]
20. Detsky AS, McLaughlin JR, Baker JP, Johnston N, Whittaker S, Mendelson RA, et al. What is subjective global assessment of nutritional status?. JPEN J Parenter Enteral Nutr. 1987;11(1):8-13. [ Links ]
21. Gondolesi G, Ramisch D, Padin J, Almau H, Sandi M, Schelotto P, et al. What is the normal small bowel length in humans? First donorbases cohort analysis. Am J Transplant. 2012;12:s49-s54. [ Links ]
22. Solar H, Doeyo M, Ortega M, De Barrio S, Olano E, Moreira E, et al. Postsurgical Intestinal Rehabilitation Using Semisynthetic Glucagon-Like Peptide-2 Analogue (sGLP-2) at a Referral Center: Can Patients Achieve Parenteral Nutrition and sGLP-2 Independency? JPEN J Parenter Enteral Nutr. 2021;45(5):1072-1082. [ Links ]
23. Fabeiro M, Dalieri M, Martínez M, Galarraga M, Prozzi M, Barcellandi P, et al. Home parenteral nutrition (HPN): feasibility of its implementation from a public hospital. Nutr Hosp. 2011;26(6):1435-9. [ Links ]
24. Ramisch D, Rumbo C, Echevarria C, Moulin L, Niveyro S, Orce G, et. al. Long-Term Outcomes of Intestinal and Multivisceral Transplantation at a Single Center in Argentina. Transplant Proc. 2016;48(2):457-62 [ Links ]
25. Abu-Elmagd KM, Armanyous SR, Fujiki M, Parekh NR, Osman M, Scalish M, et al. Management of five hundred patients with gut failure at a single center: surgical innovation versus transplantation with a novel predictive model. Ann Surg. 2019;270(4):656-74. [ Links ]
26. Gondolesi GE, Pattín F, Nikkoupur H. Management of intestinal failure in middle-income countries, for children and adults. Curr Opin Organ Transplant. 2018;23(2):212-18. [ Links ]
27. Gondolesi GE, Doeyo M, Echevarria Lic C, Lobos F, Rubio S, Rumbo C, et al. Results of surgical and medical rehabilitation for adult patients with type iii intestinal failure in a comprehensive unit today: building a new model to predict parenteral nutrition independency. JPEN J Parenter Enteral Nutr. 2020;44(4):703-13. [ Links ]
28. Bond A, Taylor M, Abraham A, Teubner A, Soop M , Carlson G, et. al. Examining the pathophysiology of short bowel syndrome and glucagon-like peptide 2 analogue suitability in chronic intestinal failure: experience from a national intestinal failure unit. Eur J Clin Nut. 2019;73(5):751-6. [ Links ]
29. Pironi L, Sasdelli AS, Venerito FM, Musio A, Pazzeschi C, Guidetti M. Candidacy of adult patients with short bowel syndrome for treatment with glucagon-like peptide-2 analogues: A systematic analysis of a single centre cohort. Clin Nutr. 2021;40(6):4065-74. [ Links ]
30. Bioletto F, D'Eusebio C, Merlo FD, Aimasso U, Ossola U, Pellegrini M, et. al. Efficacy of teduglutide for parenteral support reduction in patients with short bowel syndrome: a systematic review and meta-analysis. Nutrients 2022;14(4):796. [ Links ]
31. Gondolesi GE, Bisigniano L, Bertolotti A, Schelotto PB, Raffaele P. Organ transplantation in Argentina. Transplantation. 2018;102(6):883-6. [ Links ]
32. Norsa L, Artru S, Lambe C, Talbotec C, Pigneur B, Ruemmele F, et al. Long term outcome of intestinal rehabilitation, in children with neonatal very short bowel syndrome: Parenteral nutrition or intestinal transplantation. Clin Nutr. 2019;38(2):926-33. [ Links ]
33. Belza C, Wales PW. Management of pediatric intestinal failure related to short bowel syndrome. Semin Pediatr Surg. 2022;31(3):151-75. [ Links ]
34. Kocoshis S, Carter BA, Hill S, Horslen S, Li B, Goyal S, et al. Intestinal adaptation in children with short bowel syndrome during treatment with teduglutide. JPEN J Parenter Enteral Nutr. 2016;40:132-3. [ Links ]
35. Ramos Boluda E, Redecillas Ferreiro S, Manrique Moral O, García Romero R, Irastorza Terradillos I, Nuñez Ramos R, et al. Experience with teduglutide in pediatric short bowel syndrome: first real-life data. J Pediatr Gastroenterol Nutr. 2020;71:734-9. [ Links ]
36. Gigola F, Cianci MC, Cirocchi R, Ranucci MC, M, Coletta R, Morabito A. Use of teduglutide in children with intestinal failure: a systematic review. Front Nutr. 2022;9:866518. [ Links ]
37. Rumbo C, Martinez MI, Gondolesi GE, Fernandez A. Intestinal rehabilitation in Latin-America, report of the first paediatric case treated with Teduglutide, fifty weeks of follow up. J Pediatr Gastroenterol Nutr. 2018;66:391-2. [ Links ]
38. Martinez MI, Rumbo C, Fernández A, Ramisch D, Gondolesi GE. Teduglutide: intestinal rehabilitation in children, our initial experience. Transplantation. 2019;103:PS162. [ Links ]
39. Kaufman SS, Avitzur Y, Beath SV, Ceulemans LJ, Gondolesi GE, Mazariegos GV, et al. new insights into the indications for intestinal transplantation: consensus in the year 2019. Transplantation. 2020; 104(5):937-46. [ Links ]
40. Lacaille F, Irtan S, Dupic L, Talbotec C, Lesage F, Colomb V, et. al. Twenty-eight years of intestinal transplantation in Paris: experience of the oldest European center. Transpl Int. 2017; 30(2):178-86. [ Links ]
41. Gondolesi G. President's Message. International Intestinal Rehabilitation & Transplant Association. The Transplantation Society [Internet]. [Acceso 21 Sept. 2022]. Disponible en: https://tts.org/irta-about/irta-presidents-message. [ Links ]
Received: March 10, 2023; Accepted: September 19, 2023











 texto em
texto em