Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.115 no.4 Cap. Fed. dez. 2023 Epub 29-Nov-2023
http://dx.doi.org/10.25132/raac.v115.n4.1736
Original article
Laparoscopic liver resections: a descriptive study of our 16-year experience
1Sección de Cirugía Hepatobiliopancreática y Trasplante Hepático. Servicio de Cirugía General Hospital Privado de Comunidad. Mar Del Plata, Buenos Aires. Argentina.
Background:
Background: Improvements in laparoscopic surgery have led to more rapid progress in laparoscopic liver resections. The indications of this approach are still matter of debate.
Objectives:
The aim of the present study is to describe the results obtained in series of patients undergoing laparoscopic liver resections.
Material and methods:
We conducted a descriptive, observational and analytical study. We evaluated a series of patients undergoing laparoscopic liver resection categorized by Iwate criteria between January 2005 and October 2021. The demographic variables, diagnosis, type of approach, intraoperative findings, clinical and technical aspects and complications, were analyzed.
Results:
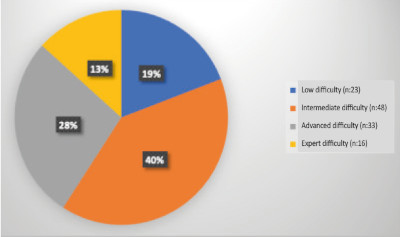
Of 159 patients undergoing hepatectomies, 120 procedures were performed laparoscopically (applicability rate 75%) and were divided into four groups according to the Iwate scoring system: low (difficulty index 0-3), 23 patients (19%); intermediate (difficulty index 4-6), 48 patients (40%);
advanced (difficulty index 7-9), 33 patients (28%); and expert (difficulty index 10-12). 16 patients (13%). Median age was 62 years. The rate of Clavien-Dindo complications ≥ grade 3 was 14.28% and mortality rate was 2.5%. In resections of colorectal liver metastases disease-free survival was 75%, 59%, 46% at 1, 3 and 5 years, respectively, and overall survival was 93%, 79% and 69%, at 1, 3 and 5 years, respectively.
Conclusions:
Laparoscopic liver resection resulted a feasible and safe technique and should be performed by trained surgeons, in specialized centers and with long learning curves to increase the applicability rate from the simplest resections to the most complex ones. Careful selection of patients is required to ensure their safety.
Keywords: video-assisted laparoscopy; hepatectomy; minimally invasive
Introduction
Laparoscopic liver surgery had a slow but continuous development. As it occurred with colorectal surgery, the initial concerns about this approach were progressively answered. However, as liver surgery is significantly different in many respects, the feasibility and usefulness of this new technique had to be tested. In 1991, Reich et al. published the first laparoscopic non-anatomic liver resection; Azagra et al. reported the first laparoscopic anatomic liver resection (left lateral sectionectomy) in 19961),(2),(3),(4.
The technique was further improved thanks to the contribution and exchange of ideas generated among the international pioneers of this technique. Several meetings were later held in 2008 in Louisville, USA, 2014 in Morioka, Japan, and 2018 in Southampton, Great Britain, where expert consensus statements and guidelines were developed. The International Laparoscopic Liver Society (ILLS) was created and organized the first congress in Paris in 2017, then in Tokyo in 2019, which greatly contributed to the safe development of this technique5),(6),(7.
The initial objections to the laparoscopic approach were due to the fact that liver surgeons had no experience in laparoscopy; therefore, the first series were performed by laparoscopic surgeons not specialized in liver surgery. This was the starting point for many surgeons trained in general laparoscopic surgery to acquire specific training in conventional and laparoscopic hepatobiliary surgery8),(9),(10.
As the interest in minimally invasive liver surgery grew, a standardized framework was needed to guide surgeons of varying expertise as to which operations were going to be more technically difficult than others. The Iwate scoring system, proposed in 2014, is a 4-level classification system of difficulties that utilizes six preoperative factors and has been validated as a tool to guide hepatobiliary surgeons with varying degrees of laparoscopic experience to identify the difficulty level of operations. When applied to laparoscopic hepatectomies, the IWATE criteria have been shown to be a reliable indicator of operative difficulty7),(9),(10),(11.
Our institution has extensive experience in performing laparoscopic surgeries. This experience dates back to 1992 with our first cholecystectomy and in 2005 we also began with laparoscopic liver surgeries. Since then, several national reports were published from centers in Buenos Aires, including a cohort between 2000 and 201912),(13),(14. Therefore, we find it relevant to present our experience at Hospital Privado de Comunidad in Mar del Plata.
The aim of the present study was to describe the results obtained in series of patients with laparoscopic liver resections.
Material and methods
We conducted an observational, descriptive and analytical study on a cohort of patients who underwent laparoscopic liver resection. The study was evaluated and approved by the institutional review board.
Our hospital provides capitated care to patients with several workers’ health insurances coverage and to those belonging to the hospital health insurance. This ensures that the population receives follow-up care in the same institution during the postoperative period. We also count with a team specialized in hepato-biliarypancreatic (HBP) surgery and liver transplantation.
The patients included were >18 years who underwent laparoscopic hepatectomy between January 2005 and October 2021. All the patients were followed up for a minimum of 6 months.
The decision on the type of approach was based on the technical difficulty, previously evaluated by means of imaging tests and intraoperative laparoscopic ultrasound.
The difficulty level of liver resections was determined using the Iwate criteria, which comprised six variables with a total score ranging from 0-12. These variables include tumor location (1-5 points), the extent of liver resection (0-4 points) tumor size < or > 3 cm (0-3 cm), proximity to a major hepatic vessel (0-1 point), liver function according to the Child-Pugh score (0-1 point) and the utilization of a hand-assisted hybrid technique (0-1 point). The 12 difficulty levels were divided into 4-level difficulty, as follows: low (0-3), intermediate (4-6), advanced (7-9) and expert difficulty (10-12)7.
We recorded the characteristics of the overall population and of the patients, the operative risk according to the American Society of Anesthesiologists (ASA) physical status classification15, tumor location, number and size of lesions to be resected, operative time, need for transfusions and intraoperative bleeding, pedicle clamping, need for conversion to open surgery, oncological outcomes, rate of R0 resections, 90-day mortality and complications according to Clavien-Dindo clasification16.
Surgical technique
All the patients were previously discussed in a multidisciplinary meeting with the participation of surgeons, specialists in HBP surgery and liver transplantation, hepatologists and oncologists. The preoperative assessment was performed by intensivists and anesthesiologists.
The patient is positioned in the supine position and the laparoscopy tower is preferably placed on the right side of the patient. The first trocar is placed near the umbilicus (right paraumbilical) using the closed technique for creating pneumoperitoneum (10-12 mm Hg). Then, 3 or 4 12-mm or 5-mm trocars, as necessary, are inserted along the operative area in the upper hemiabdomen. Laparoscopic ultrasound is performed using a 7.5 mHz probe (Aloka 3500®).
Once the type of resection has been defined, we proceed to prepare the Pringle maneuver. At the beginning of the experience, we used the extracorporeal maneuver, wrapping around the hepatic pedicle the end of a 3-4 mm-thick fabric loop. Both ends of the loop were passed through a K10 catheter and the hepatic pedicle was clamped from the outside of the abdomen. Over the last years, we replaced this technique with extracorporeal clamping using a Foley’s catheter.
We use intermittent Pringle maneuver; clamping periods of 15 minutes in normal livers or of 10 minutes in patients with chronic liver diseases (cirrhotic livers or history of chemotherapy) are separated by 5-minute periods of declamping.
After conducting laparoscopic exploration of the liver, the transection line is marked with monopolar scalpel. The initial parenchyma transection can be performed using the same electro cautery or with harmonic scalpel (Ultracision®). Beyond a depth of
2 cm, we prefer other energy-based vessel sealing devices, as Ligasure (Covidien®), Enseal (Ethicon®) or Biclamp (Medtronic®) depending on the availability. After parenchymal transection, vessels > 5 mm are controlled with LT100 or LT300 metal clips or clips of non-absorbable polymer (Hem-O-Lok®). Linear stapler is used to transect the pedicle and the suprahepatic veins but not for parenchymal transection. In left lateral sectionectomies, we prefer to extrafascial approach to section the pedicle of segments 2 and 3. In the remaining anatomic hepatectomies we use intrafascial dissection of the corresponding pedicles.
Hemostasis of the liver sectioned surface is managed with bipolar cauterization or, in certain cases, Argon gas. Biliary leaks are closed using intracorporeal sutures.
After completing the hepatectomy, the surgical specimen is put into a plastic bag and removed through a pre-existing wound, a trocar site extension, or a Pfannenstiel incision.
Results
A total of 159 liver resections were performed during the study period; 120 of them (74%) were via laparoscopy. Median age was 61 years (percentiles 25-75: 51-71) and 55.8% (n = 67) were women. The ASA physical status classification system was grade 1 and 2 in 88 (73.3%) patients and > grade 3 or greater in 32 (26.9%).
The diagnoses of benign and malignant conditions are described in Tables 1 and 2. Malignant conditions were more common (79%; n = 95); among them, colorectal liver metastases were the most prevalent (n = 68).
TABLE 1 Diagnoses of benign conditions in the series of 120 laparoscopic liver resections
| Benign conditions | n |
| Suspected malignancy | 16 |
| Caroli’s disease | 4 |
| Complicated benign cyst | 1 |
| Liver abscesses | 1 |
| Undetermined lesion due to bile duct compression | 1 |
| Secondary atrophy (hepatolithiasis) | 1 |
| Necrosis of the right anterior segment | 1 |
| Total | 25 (20.8%) |
TABLE 2 Diagnoses of malignant conditions in the series of 120 laparoscopic liver resections
| Malignant conditions | n |
| Colorectal liver metastasis | 68 |
| Hepatocellular carcinoma | 10 |
| Metastasis of brest cancer | 6 |
| Gallbladder cancer | 5 |
| Inthrahepatic cholangiocarcinoma | 3 |
| Matastasis of pancreatic cancer | 1 |
| Metastasis of gastric cancer | 1 |
| Undifferentiated sarcoma | 1 |
| Total | 95 (79.2%) |
There were 103 minor hepatectomies (45 anatomic and 58 non-anatomic liver resections) and 17 major hepatectomies, including three patients who underwent the two-stage hepatectomy technique (ALPPS). We also found 34 multiple resections (of at least two segments).
The left lateral sectionectomy was the most common anatomic resection (n = 18).
The difficulty of the procedures according to the Iwate scoring system are described in Figure 1.
Table 3 shows the outcomes and intraoperative events.
TABLE 3 Laparoscopic liver resections: intraoperative variables, 30-day mortality, morbidity and mortality, classified according to the Iwate scoring system
| Iwate (n) | 1-3 (23) | 4-6 (48) | I7-9 (33) | 10-12 (16) | Total (120) |
| Operative time in minutes, median (percentiles 25-75) | 150 (110-240) | 240 (160-340) | 300 (210-340) | 360 (300-420) | 280 (180-360) |
| Blood loss in mL, median (percentiles 25-75) | 200 (100-1000) | 500 (200-1200) | 700 (400-1200) | 800 (500-2500) | 600 (200-1200) |
| Units of packed red blood cells, median (percentiles 25-75) | 0 | 0 | 0 | 2 (0-4) | 0 |
| Hepatic pedicle clamping, n (%) | 10 (43.5) | 31 (64.6) | 21 (63.6) | 13 (81.25) | 78 (65) |
| Hepatic pedicle clamping in minutes, median (percentiles 25-75) | 35 (20-45) | 60 (30-80) | 60 (37.7-77.5) | 55 (34.5-80) | 57.5 (30-77.5) |
| Conversion rate, n (%) | 1 (4.3) | 7 (14.6) | 7 (21.2) | 6 (37.5) | 21 (17.5) |
| 30-day mortality, n (%) | 0 | 1 (2) | 0 | 2 (12.5) | 3 (2.5) |
| Clavien-Dindo grade 3 and 4, n (%) | 1 (4.34) | 2 (4.16) | 2 (6) | 3 (18.7) | 8 (6.6) |
| Length of hospital stay in days, median and interquartile range | 4 (3) | 4 (3) | 5 (4) | 7 (6) | 4 (4) |
There were 77 (98%) R0 resections due to colorectal liver metastases, and only one R1. In this group, local recurrence was 3.7% at 5 years, diseasefree survival was 75%, 59%, 46% at 1, 3 and 5 years, respectively, and overall survival was 93%, 79% and 69%, at 1, 3 and 5 years, respectively.
The conversion rate was 17.5% (n=21). Conversion was due to bleeding complications in 7 patients while 1 patient experienced intolerance to pneumoperitoneum and carbon dioxide retention. Technical difficulties and the presence of a greater number of lesions and/or unfavorable tumor location for laparoscopic resection were the causes in the remaining cases.
According to the Iwate scoring system, conversion was higher in the expert and advanced groups (Table 3). On three occasions we decided to convert laparoscopic surgery to hand-assisted surgery due to difficult bleeding control and poor exposure of the operation area in one patient, and to improve exposure and resect large lesions in segment VIII in two other patients.
Eight patients (6.6%) presented complications grade 3a, 3b and 4 of the Clavien-Dindo classification.
Three complications occurred in the expert group. Two patients had biliary fistulas, which were treated with CT-guided percutaneous drainage. Additionally, one patient required surgery after the minimally invasive treatment failed due to a collection in the surgical bed.
In the advanced and intermediate groups, there were two complications grade 3 or 4 per group.
In the advanced group, one patient presented a bleeding complication that required a new operation, and another patient, operated for a hepatocellular carcinoma, developed liver failure with encephalopathy after a left hepatectomy.
In the intermediate group, there was one patient with a percutaneously drained abscess and prolonged ileus and another with a percutaneously drained biloma and severe respiratory complications due to nosocomial infections.
In the low difficulty level of the Iwate scoring system, one patient required percutaneous ultrasoundguided drainage for an infected biloma.
One patient with multiple liver metastases from colorectal cancer received a two-stage hepatectomy known as ALPPS (Associating Liver Partition and Portal vein ligation for Staged hepatectomy). The first stage was performed laparoscopically, but due to the complexity of the resection, we had to convert to open surgery for the second stage. We conducted a right posterior sectionectomy and other non-anatomic resections in the right liver. However, the patient developed posthepatectomy liver failure and died after 25 days (small-for-size syndrome)17.
The other two deceased patients were 74 and 72 years old, and both had cirrhosis and hepatocellular carcinoma at segment 7. The first case was converted due to technical difficulty at the beginning of surgery and developed posthepatectomy liver and kidney failure, requiring hemodialysis.
The other patient had intraoperative bleeding complications that required conversion and hemostasis of a right suprahepatic collateral vein. He was hemodynamically unstable intraoperatively and postoperatively, developed multiple organ failure and died 12 hours after surgery.
None of the patients in the low and advanced difficulty groups died in the postoperative period (Table 2).
Discussion
The current series represents one of the most extensive cohorts of laparoscopic hepatectomies including 120 cases, accounting for 75% of the total hepatectomies during the study period. This reflects a high utilization rate of the method. The most treated lesions were neoplasms, including colorectal liver metastases on 95 occasions (79.1%).
This could be explained by the fact that in our center we started our experience in laparoscopic general surgery very early during our training as general surgeons. Then, as staff physicians, we gained expertise in liver transplantation, conventional liver surgery, and advanced laparoscopic liver surgery associated with the necessary support to carry out this project provided by the institution, including the acquisition of required equipment and with the high motivation and conviction of the acting team.
As we gained experience in easier surgeries or more accessible segments, we began to operate on the posterior segments, which are more complex procedures. In consequence, complications may increase, as we demonstrated by comparing morbidity according to the Iwate score for liver resections (Table 2). It is evident that the applicability of the method is still limited, but is growing: 25% according to Nguyen, similar to the figures published in Argentina. We participated in a multicenter review conducted in South America by Juan Pekolj who reported an applicability rate of 28.5%, with our country as the one with the highest surgery rate per million inhabitants in the region (6.8 laparoscopic liver surgeries per million inhabitants). However, this rate is significantly lower compared to that of other countries, such as Japan (157.8), France (67.3), Italy (54.6), and the United States (15.1)12),(13),(14),(18),(19.
The rationale for this slow development has several reasons. Unlike other abdominal organs, the liver is a large, fragile and difficult organ to be exposed without the help of the hands as in conventional surgery. Parenchymal trasection is usually associated with risk of bleeding, biliary fistula and air embolism. The initial instruments used were not safe enough to perform it.
As for oncologic resections, there was much doubt about the risk of dissemination, trocar-site tumor implantation or inadequate resections without following the oncologic principles of conventional surgery. This is why the initial series had high rates of resection of benign tumors (45% in the review by Nguyen)18),(20),(21),(22.
Laparoscopic liver resection has experienced exponential growth since the 2000s due to the expertise acquired with the technique and to improved anesthetic techniques, along with the development of more efficient and specific instruments such as energy devices for parenchymal transection and argon plasma coagulation. This has enabled to broaden the indications for this approach23),(24),(25.
The first studies published on malignancies demonstrated similar results to those of conventional surgery. The Second International Morioka Consensus Conference concluded that there were no differences in the surgical margins obtained, recurrence-free survival and overall survival between laparoscopic liver resections for cancer and open surgery26. Maurette et al. compared patients eligible for laparoscopic surgery with those who underwent conventional hepatectomy and obtained similar oncologic results13. In our laparoscopic series, more than 89% of liver resections were due to cancer, and 98% of the cases were R0 margins. Intraoperative laparoscopic ultrasound was of great help for these results. We use laparoscopic ultrasound in a very dynamic manner: initially to determine the surgical limits of the tumor, and during parenchymal transection, measuring both the lateral margin and the deep margin which is the most difficult to obtain27. In cases of tumors of the hepatic dome, we adopted the diamond technique described to improve the percentage of R0 resections28.
If the laparoscopic transducer is not available or in the case of multiple metastases, the hand-assisted technique is an option frequently used with excellent results, as reported by Barros Schelotto et al.12.
Risk factors for conversion include high body mass index (BMI), tumor size, extent of resection, resections of the posterosuperior segments and cirrhosis29. In our series, conversion rate was 17.5% (21 patients).
The learning curve is undoubtedly a slow process. Barros Schelotto et al. report an applicability rate of 23% in the initial stage and 44% in the third stage, in agreement with the interesting comparative study by Maurette et al.13.
The recommendation is to start performing resections of the anterior segments of the liver and gradually progress to anatomic segmentectomies or bisegmentectomies. Only after performing 50 basic laparoscopic surgeries, the next step is to continue with the posterior segments, major hepatectomies, repeat liver resections, and two-stage hepatectomies. Recent studies comparing the results of laparoscopic versus robotic hepatectomy conclude that the learning curve may be shorter in the robotic group, with no differences in the rest of the variables analyzed30),(31.
In conclusion, in the series here presented, laparoscopic liver resection resulted a feasible and safe technique. A specially trained multi-disciplinary team, availability of a well-equipped staff and a fully functional operating room, along with carefully selected patients, are imperative for the progress of this approach.
Referencias bibliográficas /References
1. Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gyecol 1991; 78: 956-8. [ Links ]
2. Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy - technical aspects. Surg Endosc 1996; 10: 758-761. [ Links ]
3. Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67-72. [ Links ]
4. Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804-11. [ Links ]
5. Wakabayashi G, Cherrqui D, Geller Da, Buell JF, Kaneko H, Han HS, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619-29. [ Links ]
6. Abu Hilal M, Aldrighetti L, Dagher I, Edwin B, Troisi RI, Alikhanov R, et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann Surg. 2018;268:11-8. [ Links ]
7. Kawaguchi Y, Fuks D, Kokudo N, Gayet B. Difficulty of Laparoscopic Liver Resection: Proposal for a New Classification. Ann Surg. 2018;267:13-7. [ Links ]
8. Gayet B, Cavaliere D, Vibert E, Perniceni T, Levard H, Denet C, et al. Totally laparoscopic right hepatectomy. Am J Surg. 2007;194:685-9. [ Links ]
9. Dagher I, O'Rourke, Geller DA, Cherqui D, Belli G, Gamblin TC, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856-60. [ Links ]
10. Dagher I, Proske JM, Carloni A, Richa H, Tranchart H, Franco D. Laparoscopic liver resection: results for 70 patients. Surg Endosc. 2007;21:619-24. [ Links ]
11. Krenzien F, Wabitsch S, Haber P, Kamali C, Brunnbauer P, Benzing C, et al. Validity of the Iwate criteria for patients with hepatocellular carcinoma undergoing minimally invasive liver resection. J Hepatobiliary Pancreat Sci. 2018;25:403-11. [ Links ]
12. Barros Schelotto P, Ortiz E, Montes, L, Romero P, Almanzo S, Farinelli P, et al. Experiencia inicial en hepatectomías videolaparoscópicas. Rev Argent Cirug. 2021; 113(3):326-41. [ Links ]
13. Maurette RJ, García Ejarque M, González RR, Mihura M, Bregante ML, Bogetti JD y cols. Resecciones hepáticas laparoscópicas en el tratamiento del cáncer colorrectal. Comparación con el abordaje convencional. Rev Argent Cirug 2016; 108(3): 113-119. [ Links ]
14. Pekolj J, Álvarez F, Merlo I, Sánchez Clariá R, Arbúes G, Palavecino M, et al. Resecciones hepáticas por vía laparoscópica. Indicaciones, aspectos técnicos y resultados. Rev Argent Cirug. 2015;107(3):130-7. [ Links ]
15. Saklad M. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281-4. [ Links ]
16. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-96. [ Links ]
17. Dahm F, Georgiev P, Clavien P. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005; 5(11):2605-10. [ Links ]
18. Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831-41. [ Links ]
19. Pekolj J, Sánchez Clariá R, Salceda J, Maurette RJ, Barros Schelotto P, Pierini L, et al. Laparoscopic Liver Resection: A South American Experience with 2887 Cases. World J Surg. 2020;44:3868-74. [ Links ]
20. Nicolás M, Czerwonko M, Ardiles V, Sánchez Clariá R, Mazza O, de Santibañez E, et al. Laparoscopic vs open liver resection for metastatic colorectal cancer: analysis of surgical margin status and survival. Langenbecks. Arch Surg. 2022;407(3):1113-19. [ Links ]
21. Yoon Y-I, Kim K-H, Cho H-D, Kwon J-H, Jung D-H, Park G-C, et al. Long-term perioperative outcomes of pure laparoscopic liver resection versus open liver resection for hepatocellular carcinoma: a retrospective study. Surg Endosc. 2020;34(2):796-805. [ Links ]
22. Soubrane O, Schwarz L, Cauchy F, Perotto LO, Brustia R, Bernard D, et al. A Conceptual Technique for Laparoscopic Right Hepatectomy Based on Facts and Oncologic Principles: The Caudal Approach. Ann Surg. 2015;261(6):1226-31. [ Links ]
23. Ciria R, Cherqui D, Geller DA, Briceno J, Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann Surg. 2016; 263(4):761-77. [ Links ]
24. Tomassini F, Scuderi V, Colman R, Vivarelli M, Montalti R, Troisi RI. The single surgeon learning curve of laparoscopic liver resection: A continuous evolving process through stepwise difficulties. Medicine (Baltimore) 2016; 95(43): e5138. [ Links ]
25. Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009; 250(5): 772-82. [ Links ]
26. Wakabayashi G, Cherqui D, Geller DA, Han H-S, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21(10):723-31. [ Links ]
27. Cuesta MA, Meijer S, Paul MA, de Brauw LM. Limited laparoscopic liver resection of benign tumors guided by laparoscopic ultrasonography: report of two cases. Surg Laparosc Endosc. 1995;5(5):396-401. [ Links ]
28. Cipriani F, Shelat VG, Rawashedh M, Francone E, Aldrighetti L, Takhar A, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg. 2015; 221(2):265-72. [ Links ]
29. Ratti F, D'Alessandro, Cipriani F, Giannone F, Catena M, Aldrighetti L. Influence of body habitus on feasibility and outcome of laparoscopic liver resections: a prospective study. J Hepatobiliary Pancreat Sci. 2016;23(6):373-381. [ Links ]
30. Zhu P, Liao W, Ding Z-Y, Chen L, Zhang W-G, Zhang B-X, et al. Learning Curve in Robot-Assisted Laparoscopic Liver Resection. J Gastrointest Surg. 2019;23(9):1778-87. [ Links ]
31. Lafaro KJ, Stewart C, Fong A, Fong Y. Robotic Liver Resection. Surg Clin North Am. 2020; 100(2):265-81. [ Links ]
Received: February 03, 2023; Accepted: June 20, 2023











 texto em
texto em