Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.115 no.4 Cap. Fed. dez. 2023 Epub 12-Out-2023
http://dx.doi.org/10.25132/raac.v115.n4.1679
Scientific letter
Liver resection for the treatment of complex bile duct injury
1Unidad de Cirugía Hepatobiliar Compleja y Trasplante Hepático. Hospital El Cruce. Buenos Aires. Argentina.
Bile duct injury represents a serious health problem, with its incidence doubling since the advent of laparoscopic cholecystectomy. In cases of complex lesions affecting the hepatic confluence, association with vascular injuries, hepatic atrophy, cholangitis or failed repair, treatment is often challenging. We report the case of a female patient with a complex bile duct injury due to vascular involvement of the right hepatic pedicle who developed right liver atrophy. In the presence of septic complications, hepatectomy was necessary, along with a definitive repair of the biliary tract, resulting in positive outcomes.
Keywords: bile duct injury; sepsis
Bile duct injury (BDI) is associated with significant morbidity and reduction in quality of life and long-term survival. Despite advancements in surgical skills, imaging tests and management, the incidence of BDI remains high, particularly, and more serious, during laparoscopic cholecystectomy compared to open surgery1. Management includes minimally invasive percutaneous or endoscopic procedures, or surgical repair with bilio-digestive bypass. The indication of liver resection is rare. Management in specialized centers is essential, as bad initial decisions can have serious medical and legal consequences1.
We report the case of a 30-year-old female patient with a history of laparoscopic cholecystectomy due to acute cholecystitis. We were unable to contact the surgical team, so the information obtained is solely from the operation note. According to the report, the procedure was long and difficult, and because the Calot triangle could not be identified, the procedure was converted to open surgery. On exploration, a biliary leak was observed because of a complete bile duct section at the level of the hepatic confluence. Fine drains were placed in the right and left hepatic ducts and common bile duct, and an accessory drain was placed on the Morrison’s pouch. During the first days following the surgery, the bile output through the intrahepatic drains was 400 mL per day, while 100 mL per day was released through the common bile duct drain, and 50 mL daily through the drain positioned in the Morrison’s pouch. On postoperative day 5, the patient started with fever. A computed tomography (CT) scan revealed a liver abscess in segment VII requiring percutaneous drainage. Klebsiella pneumoniae carbapenemaseproducing bacteria was isolated.
The patient was referred to our center on postoperative day 13, hemodynamically stable and without fever. Her performance status was deteriorated and she complaint of asthenia, anorexia and loss of 5 kg. The drain tube placed in the Morrison’s pouch had been removed. The tubes from the hepatic ducts and common bile duct drained 200 mL of biliary fluid in total, and the percutaneous drainage of the hepatic abscess drained 200 mL of purulent biliary fluid. On admission, the results of the laboratory tests were white blood cell count 11,600/mm3, AST 117 IU/L, ALT 214 IU/L, alkaline phosphatase 523 IU/L, total bilirubin 6.7 mg/dl, direct bilirubin 5 mg/dl and prothrombin time 90%. We performed a CT scan that demonstrated the presence of liver abscesses in segments VII and VIII and a hilar collection associated with an injury of the right hepatic artery and the anterior branch of the right portal vein. Considering the presence of intermediate collections at the level of the hilum, we decided to replace the drains with larger multipurpose drain tubes. During hospital stay, the drainage in the abscess of segment VII and the fine drain in the common bile duct showed a progressive decrease in output until they were finally removed. The output from each biliary drain was 400 mL a day. The patient showed favorable progress and her nutritional status improved. A new CT scan performed 6 months after surgery showed atrophy of the right lobe, hypertrophy of the left lobe and absence of new abdominal fluid collections (Fig. 1A-B). A magnetic resonance cholangiopancreatography showed complete bile duct section at the level of the hepatic confluence corresponding to a Strasberg type E4 injury and a Hannover type D3 injury (Fig. 1C). The output from the right drain started to decrease gradually until it reached a volume of 200 ml per day. Due to the COVID-19 pandemic, the surgery was postponed until 8 months after surgery. Laparotomy was performed through the scar of the previous incision site. The path of the drains was dissected up to the point of the pedicle, and the drainage catheters were identified in both hepatic ducts. There was right lobe atrophy and compensatory left lobe hypertrophy (Fig. 2A-B). Selective cholangiography through the right hepatic duct revealed complete loss of normal anatomy (Fig. 2C), whereas imaging through the left hepatic duct showed no damage to the corresponding biliary tree. We decided to perform right liver resection. Parenchymal transection was done using harmonic scalpel and ultrasonic surgical aspiration. Finally, Roux- en-Y hepaticojejunostomy was constructed and sutured with polupropylene 6/0 (Hepp Couinaud approach) (Fig. 2D). The patient was discharged on postoperative day 6. Bile culture was positive for Citrobacter susceptible to ciprofloxacin. The pathology report of the surgical specimen demonstrated mixed inflammatory infiltrate with proliferation of small bile ducts and fibrosis. A core needle biopsy of the left lobe resulted in mixed inflammatory infiltrate. After 10-month follow-up, the patient was free of complications.
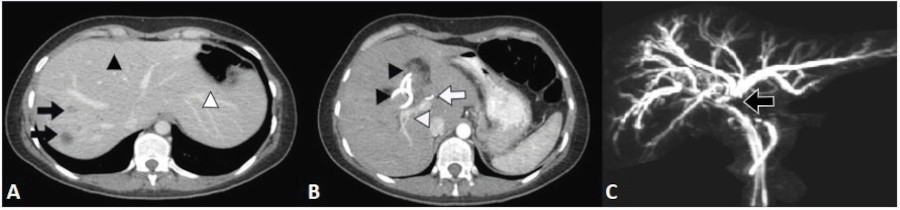
FIGURE 1 Computed tomography scan performed at 6 moths. A: Atrophy of right hepatic segments (black arrows), hypertrophy of segment IV (black arrowhead) and left lateral segment (white arrowhead). B: Percutaneous drainage catheters (black arrowhead), posterior branch of right portal vein (white arrowhead), left hepatic artery (white arrow). C: Magnetic resonance cholangiopancreatography. Strasberg type E4 and Hanover type D3 bile duct injury (black arrow).
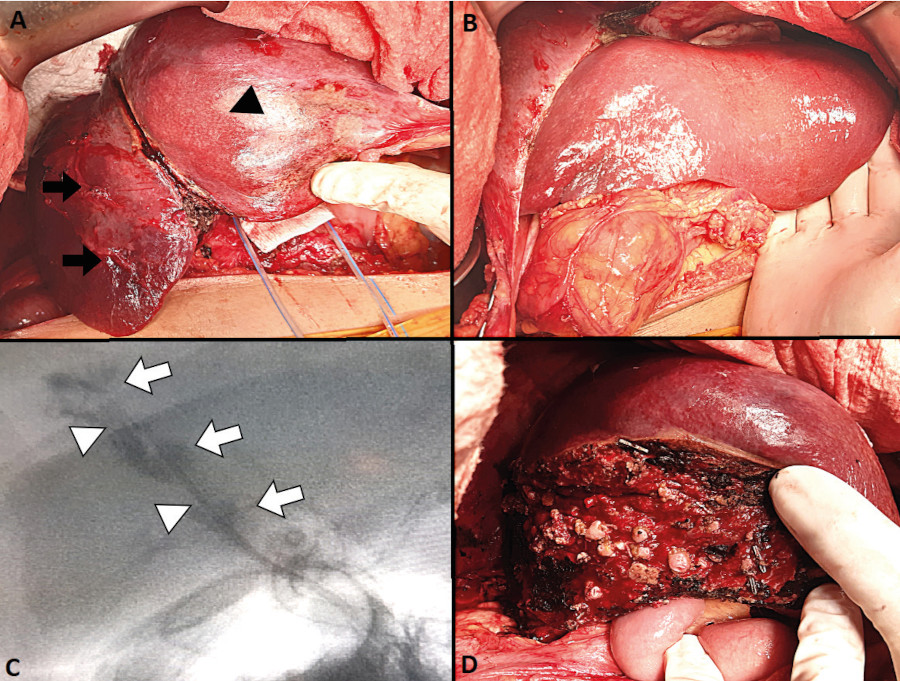
FIGURE 2 A: Right liver atrophy (black arrows) and hypertrophy of segment IV (black arrowhead). B: Hypertrophy of the left lateral segment. C: Right bile duct, areas of strictures (white arrowhead) and dilatations (white arrow). D: Bed transection and hepaticojejunostomy.
Bile duct injuries type E1-E3 of the Strasberg classification can usually be repaired through minimally invasive percutaneous or endoscopic procedures, or by performing a bilio-digestive anastomosis. The management of complex injuries is controversial. The combination with vascular injuries is more common in proximal Strasberg type E4-E5 injuries, where the right hepatic artery is the most affected element due to its proximity to the common hepatic duct (12-47%). Vascular involvement produces areas of ischemia, with risk of superinfection and abscess development between the first and third week. If there is also bile duct stricture, parenchymal atrophy may develop in the long term. Liver resection has a high efficacy (60-90%) to manage the symptoms related to these complications. However, morbidity ranges between 50 and 61%, significantly higher than that observed after hepatectomy for other indications3. Although early hepatectomy has been described as a treatment for BDI with vascular involvement that leads to hepatic necrosis with septic foci such as abscesses or biliary leaks, our recommendation is against this indication due to its high mortality rate of almost 50%, as reported by Strasberg et al.2. Delayed hepatectomy may be considered for vascular injuries that result in infected necrosis or symptomatic lobar atrophy. It is also indicated for intrahepatic bile duct strictures that are spreading or unresponsive to dilatation treatments4. It is a technically complex procedure because of the widespread fibrosis affecting the hepatic hilum, which hinders the identification of pedicle elements. The recommendation is to perform hepatectomies during long-term follow-up to improve both local and general conditions, which allows for bilio-digestive bypass, and after an accurate evaluation of irreversible ischemic liver atrophy3. The aim of liver resection is to remove fibrotic and atrophic liver parenchyma and the diseased biliary confluence for preventing progressive liver damage and potential malignancy caused by bile stasis and repeated cholangitis5.
Successive failures of therapeutic procedures may determine the manifestation of late complications such as portal hypertension and secondary biliary cirrhosis. In these cases, liver transplantation remains the only definitive treatment with favorable outcomes regarding long-term survival and quality of life. However, it presents increased immediate morbidity and m ortality6.
In our patient, we initially treated the infection foci and optimized the management of fistulas with percutaneous drainage. When we replaced the intrahepatic drains with percutaneous catheters, drainage reached an optimal level. When biliary fluid stopped draining from the common bile duct, the catheter was removed. Parenchymal atrophy developed as the biliary output through the corresponding drain decreased. Delayed management enabled us to control local complications, assess the degree and extent of atrophy, and improve our patient’s general condition. Finally, we decided to perform a right liver resection due to the presence of a Strasberg type E4/Hannover type D3 BDI that involved the arterial and portal vessels, symptomatic lobar atrophy (abscesses) and loss of bile duct integrity, which was reconstructed by performing a Roux-en-Y hepaticojejunostomy.
We emphasize the importance of managing complex BDIs in specialized centers that offer multiple therapeutic options, including hepatectomy and ultimately transplantation.
Referencias bibliográficas /References
1. Pekolj J, Yanzón A, Dietrich A, Del Valle G, Ardiles V, De Santibáñes E. Major liver resection as definitive treatment in post-cholecystectomy common bile duct injuries. World J Surg. 2015;39(5): 1216-23. [ Links ]
2. Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford). 2011;13(1):1-14. [ Links ]
3. Li J, Frilling A, Nadalin S, Broelsch CE, Malago M. Timing and risk factors of hepatectomy in the management of complications following laparoscopic cholecystectomy. J Gastrointest Surg. 2012; 16(4):815-20. [ Links ]
4. Laurent A, Sauvanet A, Farges O, Watrin T, Rivkine E, Belghiti J. Major hepatectomy for the treatment of complex bile duct injury. Ann Surg. 2008; 248(1):77-83. [ Links ]
5. Jablonska B. Hepatectomy for bile duct injuries: when is it necessary? World J Gastroenterol. 2013; 19(38):6348-52. [ Links ]
6. Ardiles V, McCormack L, Quiñonez E, Goldaracena N, Mattera J, Pekolj J, Ciardullo M, de Santibáñes E. Experience using liver transplantation for the treatment of severe bile duct injuries over 20 years in Argentina: results from a National Survey. HPB (Oxford). 2011; 13(8):544-50. [ Links ]
Received: May 03, 2022; Accepted: July 25, 2022; pub: October 12, 2023











 texto em
texto em 



