Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.115 no.4 Cap. Fed. dez. 2023 Epub 18-Out-2023
http://dx.doi.org/10.25132/raac.v115.n4.1698
Scientific letter
Glomus tumor of the small bowel
1Servicio de Cirugía General. Clínica CMIC. Neuquén, Argentina.
2Servicio de Clínica Médica. Clínica CMIC. Neuquén, Argentina.
Glomus tumors (GTs) are benign vascular neoplasms of soft tissues that occur in peripheral acral areas. Most intra-abdominal GTs develop in the stomach; the incidence of intestinal presentation is very low, We report the case of a 26-year-old female patient with chronic mild pain in the umbilical region with no other symptoms or signs. The imaging tests demonstrated a heterogeneous abdominopelvic mass with a solid vascularized component, and a cystic component with papillary projections.
The pathology examination of the surgical specimen reported the diagnosis of GT of the small bowel (SB). We conducted a review of the literature and found their low incidence, affecting both men and women between 26 and 88 years without a predominant geographic distribution.
Glomus tumors should be considered as a rare differential diagnosis in the presence of small SB tumors.
Keywords: paraganglioma extra adrenal; retroperitoneal neoplasm
Glomus tumors (GTs) or glomangiomas are rare benign vascular neoplasms that arise from modified smooth muscle cells of the glomus body surrounding the arteriovenous anastomosis involved in thermoregulation and peripheral blood flow (canals of Sucquet-Hoyer)1,2.
These tumors are extremely rare, accounting 2% of smooth tissue neoplasms3. They are usually found in peripheral areas, particularly in subungual regions of the fingers and the deep dermis of hands and feet. Extracutaneous involvement is exceptional, < 2.5%4, and includes the stomach, intestines, peritoneum, mediastinum, lung, pancreas, urinary bladder, and vagina2. Most intra-abdominal GTs have been reported to occur in the stomach; the incidence of intestinal presentation is very low5 and those specifically involving the ileum are extremely rare3.
An otherwise healthy 26-year-old female patient presented with chronic abdominal pain lasting 2 months. The pain was dull, mild, and constant, situated in the umbilical region with radiation to the hypogastrium, and was not related to any specific triggers. The patient did not complain of weight loss, fever, or urinary, gynecological or respiratory symptoms. As the pain intensified, she consulted a clinician.
Her performance status was good, and she was fully conscious, alert, oriented and hemodynamically stable. She had adequate perfusion, and there were no signs of heart failure. Skin color, temperature and moisture were normal. The patient was breathing well, and breath sounds were normal. On palpation, the abdomen was flat, soft, depressible, and non-tender with normal bowel sounds and no visceromegaly. On rectal examination, the tone of the anal sphincter was normal, the walls of the anal canal were smooth and elastic, the rectal ampulla was empty, and the gloved finger was clean. The laboratory tests reported normal complete blood count, metabolic and immunological profiles, and proteins.
The patient underwent pelvic ultrasound with transabdominal and transvaginal exploration. A rounded, heterogeneous mass measuring 7 × 5.7 × 4 cm was visualized towards the bottom of the Douglas’ pouch. The mass had a solid, hypervascularized component, and acysticcomponent with heterogeneous papillary projections towards its interior. Despite the mass was between both ovaries, no connection with them could be identified. The workup progressed with a magnetic resonance imaging (MRI) using a contrast agent. Both ovaries were correctly positioned and displayed preserved size and morphology. A solid-cystic mass was adjacent to the small bowel (SB) loops. The solid component exhibited strong enhancement in the arterial phase, with blood flow presumably supplied by branches of the mesenteric artery. A positron emission tomography/computed tomography (PET-CT) with injection of 10 mCi of fluorine-18 fluorodeoxyglucose (18F-FDG) was ordered. There was a solid-cystic mass measuring 6.32 × 5.92 × 5.8 cm in the abdominopelvic region (Fig. 1); the solid component had moderate marker uptake with maximum standardized uptake value (SUVmax) of 2.20. The mass displaced of the urinary bladder and the adjacent bowel loops.
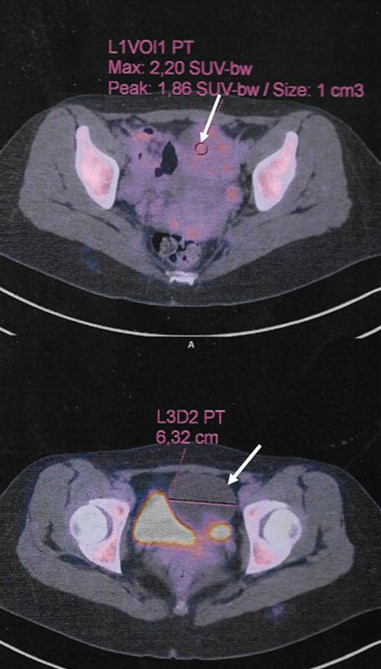
FIGURE 1 Positron emission computed tomography. The arrow shows the abdominopelvic mass with SUVmax of 2.20.
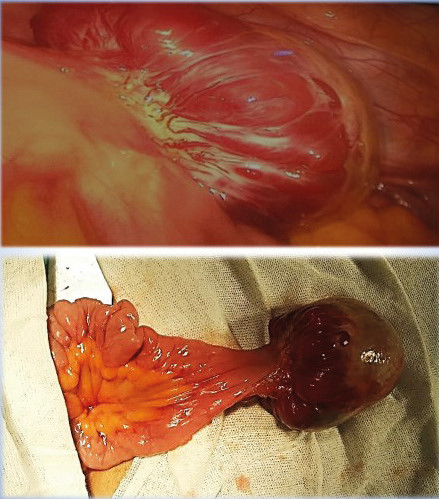
A diagnostic laparoscopy was decided to determine the origin of the tumor. On exploration, a nodular mass of approximately 6.5 cm in diameter was identified in the antimesenteric border of the small bowel (Fig. 2), 40-50 cm from the ileocecal valve. In view of a suspected gastrointestinal stromal tumor (GIST), conversion was decided. The small bowel segment involved was resected and an end-to-end intestinal anastomosis was performed through a Pfannenstiel incision. The patient was hospitalized for one week and then resumed her regular physical and work activities, along with a balanced diet 40 days after the operation. Since then, she has been monitored for 2 years without any symptoms.
The pathology report confirmed the presence of a glomus tumor in the SB, with clear margins. The histological analysis revealed a distinct tumor within the SB wall in the muscularis propria, demonstrating predominantly extramural growth. The tumor consisted of a proliferation of rounded cells with round nuclei and
abundant clear cytoplasm, without atypia. The tumor cells were arranged surrounding congestive vessels, and there were some hemangiopericytes, areas of necrosis, collagen accumulation, and bleeding, but no mitoses or pleomorphism. The serosa and muscularis propria layer adjacent to the tumor showed spaces lined by endothelium with cystic dilation. The submucosa beneath the tumor exhibited abundant dilated and ectatic vessels.
Immunohistochemical staining showed positive staining for vimentin and alpha smooth muscle actin (α-SMA) and negative staining for other markers (desmin, CK7, PAX-8, HMB.45, chromogranin and synaptophysin); CD34 and CD31 highlights endothelial cells, and c-KIT identifies mast cells.
Glomus tumors are usually benign neoplasms, but occasionally display unusual features such as large size, infiltrative growth, necrosis, nuclear polymorphism and mitotic activity3,5. Extracutaneous presentations are rare. Within the gastrointestinal tract, the stomach is the most frequent site of involvement, while GTs of the small intestine are extremely rare.
The incidence of primary gastrointestinal tumors in the small intestine is very low. Several studies, including our case, have reported patients ranging in age from 26 to 88 years2,3,4,5,6, and there are no reports in pediatric patients. Men and women are affected in a 1:1 ratio; many cases have been reported in 7 countries, with no predominant geographic distribution.
Glomus tumors in the small intestine can be asymptomatic and detected incidentally during an ultrasound examination. They can also cause symptoms such as abdominal pain, gastrointestinal bleeding (hematemesis, melena, or hematochezia), or symptoms associated with an intra-abdominal mass (abdominal bloating, vomiting, or constipation). The workup includes ultrasonography, gastrointestinal endoscopy, computed tomography, magnetic resonance imaging, and positron emission tomography. In one case, suspicion of rectal cancer metastasis prompted a direct laparotomy5.
Due to their low incidence, it cannot be confirmed that there is a preferred location within any specific part of the small bowel (36.4% in the duodenum, 18.2% in the jejunum, and 45.4% in the ileum). Partial resection of the jejunum or ileum, either through laparotomy or laparoscopy, is the usual treatment. Duodenal tumors may require pancreaticoduodenectomy5. For patients with comorbidities, embolization of the gastroduodenal artery or endoscopic mucosectomy are reported alternatives5.
The preoperative diagnosis was difficult and was only made by endoscopic biopsy in one case prior to surgery. In other cases, the preoperative diagnosis was submucosal tumor, carcinoid tumor or neuroendocrine tumor, or GIST (as in our case).
The differential diagnoses of GTs of the small intestine included other cytologically related tumors such as GIST, carcinoid tumors, hemangiopericytomas, paragangliomas,andlymphomas.Immunohistochemical tests can rapidly characterize tumor profile. Glomus tumors are almost always positive for alpha-smooth muscle actin (α-SMA), vimentin and collagen type IV3,5, and negative for CD34, c-KIT, cytokeratin, chromogranin and synaptophysin.
Therefore, GTs should be considered as a rare differential diagnosis in the presence of SB tumors. They are usually benign and solitary; however, as there is no information on long-term follow-up, periodic monitoring is recommended to identify early symptoms of disease.
Referencias bibliográficas /References
1. Giraldo C, Papasidero S, Pedernera A, Toledo G, Bertolini C, Rillo O. Tumor glómico. Rev Arg Reumatol. 2012;23(1):38-42. [ Links ]
2. De Bruin A, Verhoef C, Den Bakker M, Van Geel A. Glomus tumor of the mesentery with atypical features: a case report. Int J Surg Pathol 2008;16(4): 440-2. [ Links ]
3. Abu-Zaid A, Azzam A, Amin T, Mohammed S. Malignant glomus tumor (glomangiosarcoma) of intestinal ileum: a rare case report. Case Rep Pathol. 2013; 2013:305321. doi: 10.1155/2013/305321. Epub 2013 Apr 8. [ Links ]
4. Campana JP, Goransky J, Mullen EG, Palavecino EM. Intestinal benign glomus tumor: description and review of the literatura. Dig Dis Sci. 2014; doi: 10.1007/s10620-014-3172-9. [ Links ]
5. Fukui J, Inoue k, Mukaide H, Ozaki G, Michiura T, Tokubara K, et al. A case of small intestine glomus tumor detected by obscure gastrointestinal bleeding of indeterminate origin. Jpn J Gastroenterol Surg. 2014;47(4):223-9. [ Links ]
6. Tarangelo NP, Ha K, Skole KS. Duodenal glomus tumor: a rare cause of upper GI bleeding. Clin Gastroenterol Hepatol. 2016;14(10): e123-e124. [ Links ]
Received: February 16, 2022; Accepted: June 21, 2022; pub: October 18, 2023











 texto em
texto em