Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista argentina de cirugía
versão impressa ISSN 2250-639Xversão On-line ISSN 2250-639X
Rev. argent. cir. vol.116 no.1 Cap. Fed. mar. 2024 Epub 26-Fev-2024
http://dx.doi.org/10.25132/raac.v116.n1.1767
Original article
Conversion therapy in gastric cancer: experience at Instituto Nacional de Cancerología de Colombia
1Instituto Nacional de Cancerología. Colombia.
Background:
Gastric cancer (GC) represents a public health problem in Colombia and worldwide. Since most patients are at advanced stages at the time of diagnosis. it is necessary to develop management strategies as conversion therapy (CT).
Objective:
The aim of this study was to estimate the results of CT for treating patients with advanced and GC at Instituto Nacional de Cancerología de Colombia (INC).
Material and methods:
We included patients with incurable gastric cancer who underwent induction chemotherapy and intended curative surgery between 2010 and 2021. The clinical and pathological data and survival of the patients included were retrospectively reviewed. Overall survival (OS) was calculated from the time of initiation of chemotherapy until the date of death. Survival functions were estimated using the life table and Kaplan-Meier methods. and survival curves at 3 and 5 years were constructed.
Results:
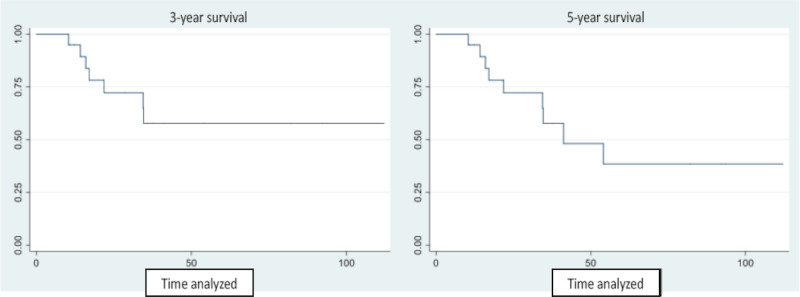
23 patients were analyzed; mean age was 56 years. and 17 (74%) were men. The most common criterion indicating unresectability was a T4b tumor in 13 cases (56.5%). All the patients underwent CT. Median follow-up was 28 months. Eleven patients developed disease recurrence (52%). Median survival was 41.2 months. and 3- and 5-year OS was 57.7% and 38.5%. respectively.
Conclusions:
CT provided an acceptable OS rate for selected patients with incurable advanced GC. This strategy requires an adequate selection of patients and multidisciplinary management in reference oncology centers.
Keywords: gastric cancer; chemotherapy; adjuvant surgery; conversion therapy; Stage IV gastric cancer
Introduction
Gastric cancer (GC) is the fifth most common malignant tumor and the fourth leading cause of cancer death worldwide. In Colombia. 8214 new cases of GC and 6491 deaths from this disease were recorded in 2020. becoming the neoplasm with the highest mortality rate. accounting for 11.7% of all cancer deaths in the country1. Most patients with GC have symptoms of an advanced stage at the time of presentation. and 34% have distant metastases at the initial presentation2. Systemic therapy and palliative care are the mainstays of treatment for patients with locally advanced GC who are not candidates for surgery or have distant metastases (also known as incurable GC3). These patients typically have a median overall survival (OS) of no more than 16 months4. However. in recent years conversion therapy (CT). defined as a surgical treatment aimed at achieving R0 resection after a favorable response to induction chemotherapy. has been gaining acceptance as a novel strategy for improving the oncologic outcomes of patients for tumors that were originally considered unresectable for technical and/or oncological reasons4. In this context. the concept of adjuvant surgery arises. defined as resective surgery performed after systemic chemotherapy and that is expected to be curative5.
The biology of gastric carcinomas varies depending on the site of metastasis and the specific presentation and behavior of the metastatic disease. Nowadays. there is no agreement on the criteria for selecting patients eligible for CT. nor on the ideal chemotherapy regimen or the optimal time interval between chemotherapy and surgery6.
Although CT looks promising. it has not been proven in controlled clinical trials probably due to its complicated nature. still under development. and the fact that the number of candidate patients is too small to carry out a randomized control trial7. The information about the efficacy of CT for the management of patients with advanced GC comes from retrospective singlecenter studies involving patients with a variety of noncurative factors. and from multicenter prospective or retrospective cohort studies focused on patients with one non-curative factor4.
The aim of the present study was to estimate the results of CT in a series of patients with advanced and incurable gastric cancer treated at the Department of Gastrointestinal Surgery of Instituto Nacional de Cancerología de Colombia (INC). over a 12-year period.
As far as we know. this would be the first cohort of CT in GC reported in Colombia.
Material and methods
Patients who underwent induction chemotherapy and subsequent intended curative surgery between January 1. 2010. and December 31. 2021. were included in the study. The study included patients who met the following eligibility criteria: 1) histologically confirmed primary gastric adenocarcinoma. and 2) stage T4b tumor. i.e.. a tumor considered incurable due to invasion of adjacent structures or organs. extra-regional lymph node involvement. or distant metastasis documented by staging investigations.
We included only patients with unresectable tumors that were locally advanced or had lymph node involvement or hematogenous metastasis and excluded those with peritoneal dissemination because they express a different tumor biology and are managed by a specific team in our institution.
Contrast-enhanced computed tomography scan of the chest and abdomen and laparoscopy were the only methods used for patient staging. Patients with Siewert type I and II gastroesophageal junction cancer were excluded. because neoadjuvant therapy is the standard of care in this setting. Additionally. patients in whom an R0 resection was not achieved were excluded from the survival analyses. as they did not receive curative surgeries. constituting a failure of CT.
We reviewed the electronic medical records of our institution from the platform SAP® and retrieved the patients’ demographic and clinical characteristics. The Eastern Cooperative Oncology Group (ECOG)8 score was used to assess patients’ performance status. The following variables were included: induction chemotherapy. type of surgery. lymph node clearance. pathology findings obtained from the surgical specimen. complications and postoperative mortality at 30 and 90 days using the Clavien-Dindo9 classification. adjuvant chemotherapy. OS rate at 3 and 5 years. and tumor recurrence.
Normality of distribution of variables was assessed using the Shapiro-Wilk test. Quantitative variables with normal distribution were expressed as mean ± standard deviation and those with non-normal distribution as median and interquartile range. and 95% confidence intervals were determined. Categorical variables were expressed as absolute and relative frequencies.
Overall survival was calculated from the time of initiation of chemotherapy until the date of death from any cause. Patients lost to follow-up or those who did not experience the event before the study ended were right censored. Survival functions were estimated using the life table and Kaplan-Meier methods. and survival curves at 3 and 5 years were constructed. All the statistical calculations were performed using Stata 16 software package. The study protocol was approved by the institutional review board o the INC.
Results
Twenty-three patients with incurable advanced GC due to invasion of adjacent organs. extra-regional lymph node involvement or hematogenous metastases were managed with CT during the study period. The response to CT was evaluated in all the patients using contrast-enhanced computed tomography before surgery. As 2 cases were excluded from the survival analyses because they did not achieve R0 resections. the final cohort was made of 21 patients. Mean age at the moment of diagnosis was 56 years (range 42-72). 74% were men and 20 patients (96%) had an ECOG score8 of 0 or 1. One-third of cases had a distal tumor. Loss of MMR proteins on immunohistochemical testing. as a surrogate marker for microsatellite instability. was found in 13% of tumors.
The most common criterion indicating unresectability was a T4b tumor. which was present in 56.5% of cases. Imaging tests confirmed unresectability in 39.1% of patients. while laparoscopy/laparotomy confirmed it in the remaining 60.9%. In 10 (43.5%) patients. surgical staging was performed at the INC.
Median time between the end of induction chemotherapy and surgery was 48 days. Total gastrectomy was performed in 52.2% of the patients. and 87% underwent D2 lymphadenectomy. with a mean of 34.3 resected nodes.
The pathology examination revealed that only 2 (8.7%) patients achieved a pathologic complete response (pCR) after induction chemotherapy. One of these patients also received preoperative radiotherapy (50 Gy). The two patients with R1 resections (8.7%) were: 1) a 42-year-old man with total gastrectomy and en bloc central pancreatectomy with positive esophageal margin. who died on postoperative day 23. and 2) a 70-year-old man with total gastrectomy plus cholecystectomy with positive gallbladder margin. who was still alive at the time the study ended (23 months). Other surgical and pathological aspects are detailed in Table 1.
TABLE 1 Postoperative and pathological outcomes of 23 patients with conversion therapy for advanced gastric cancer
| Characteristics | n | % |
|---|---|---|
| Histological type | ||
| Intestinal type | 16 | 69.7 |
| Diffuse type | 5 | 21.7 |
| Mixed type | 1 | 4.3 |
| Unclassified | 1 | 4.3 |
| HER2‡ | ||
| Yes | 3 | 13 |
| No | 17 | 73.9 |
| Unavailable data | 3 | 13 |
| Criterion to consider CT | ||
| T4b | 13 | 56.6 |
| T4b with distant lymph node involvement | 2 | 8.7 |
| Only distant lymph node involvement | 4 | 17.4 |
| Only liver metastases | 3. | 13 |
| Liver metastases with distant lymph node involve | 1 | 4.3 |
| Surgical technique | ||
| Total gastrectomy | 12 | 52.2 |
| Sub-total gastrectomy | 3 | 13 |
| Total gastrectomy with resection of adjacent organs | 6 | 26.1 |
| Subtotal gastrectomy with resection of adjacent organs | 2 | 8.7 |
| Type of lymph node dissection | ||
| D1 | 2 | 8.7 |
| D1+ | 1 | 4.3 |
| D2 | 20 | 87 |
| ypStage¶ (21**) | ||
| I | 3 | 14.5 |
| II | 6 | 28.5 |
| III | 12 | 57 |
*Siewert type III gastroesophageal junction cancer. subcardial and body gastric cancers. †Pyloric antrum cancer. ‡HER2 overexpression (ErbB2 gene). ¶AJCC 8 ed.. 2017 - classification after neoadjuvant therapy. Excluding the two patients who achieved pCR.
Platinum- and fluoropyrimidine-based chemotherapy were the most used induction regimens (Table 2).
TABLE 2 Chemotherapy regimens used in 23 patients as conversion therapy for advanced gastric cancer
| Chemotherapy | n | % |
|---|---|---|
| Cisplatin + 5-Fluorouracil | 3 | 13 |
| Cisplatin + 5-Fluorouracil + Leucovorin | 1 | 4.3 |
| Docetaxel + Cisplatin + 5-Fluorouracil | 2 | 8.7 |
| Cisplatin + Capecitabine | 4 | 17.4 |
| Carboplatin + Capecitabine | 1 | 4.3 |
| Capecitabine + Oxaliplatin | 5 | 21.7 |
| Docetaxel + Oxaliplatin + Leucovorin + 5-Fluoruracil | 5 | 21.7 |
| Capecitabine + Oxaliplatin + Trastuzumab | 1 | 4.3 |
| Folinic acid + 5-Fluorouracil + Oxaliplatin | 1 | 4.3 |
Regarding postoperative outcomes. 10 (43.5%) patients experienced complications within 90 days after surgery: two patients died and 4 patients presented complications grade IIIb or greater according to the Clavien-Dindo classification9.
In our series. 18 patients (78.2%) received postoperative chemotherapy. During follow-up. tumor recurrence was documented in 11 cases. with 6 of them occurring within 6 months of surgical resection.
Recurrences were identified in the liver. bones. peritoneum. lymph nodes and soft tissues.
After a median follow-up of 28 months (range 9-115 months). 52.2% (12) of the patients were alive. 10 had died (43.5%) and one was lost to follow-up (4.3%). Median survival in this series was 41.2 months. and 3- and 5-year OS was 57.7% and 38.5%. respectively (Fig. 1).
Discussion
This paper shows the results of CT for the treatment of unresectable GC in a series of patients in a Latin American cancer center. Considering that these patients would typically receive palliative chemotherapy or best supportive care. this therapeutic approach allows for curative treatment and improves the prognosis of a very select group of cases. Median survival in this series was 41.2 months. higher than that reported by the two Latin American series available in the literature published by Ramos et al.10 with 11.3 months and by Gallardo-Rincón et al.11 with 13 months. Overall survival at 3 and 5 years is lower when we compare it with the majority of Asian series. as the one by Sato et al.12 (75.4% at 3 years). but higher than that reported in the Italian series by Morgagni et al.13 (39.4%). However. it is difficult to compare the results. as not all the studies provide the same data (Table 3)10-40.
TABLA 3 Case series of conversion therapy in gastric cancer
| Year | Reference | Number of patients undergoing CT | Most common criterion of unresectability | ≥ D2 | R0 n(%)* | Median follow-up of patients undergoing CT† (months) | Median survival (months)* | OS* |
|---|---|---|---|---|---|---|---|---|
| 1997 | Nakajima et al. 14 | 19 | PAN/N3 | NE | 9 (47%) | NE | NE | 55.6%¶ |
| 2000 | Gallardo-Rincón et al.11 | 10 | NE | 50% | 7 (70%) | NE | 13.3 | NE |
| 2002 | Yano et al.15 | 14 | Peritoneum | NE | 8 (57%) | NE | NE | NE |
| 2010 | Suzuki et al.16 | 20 | PAN/N3 | NE | 11 (55%) | 32.2 | NE | 80%‡ |
| 54.9%§ | ||||||||
| 2012 | Satoh et al.17 | 44 | Peritoneum | 82% | 26 (59%) | > 24 | 19.2 | 75%‡ |
| 2012 | Kanda et al.18 | 28 | PAN/N3 | 96.3% | 26 (93%) | 37.5 | 29 | 49.5%§ |
| 2013 | Han et al.19 | 34 | PAN/N3 | NE | 26 (76%) | 22.7 | 22.9 | 41.4%§ |
| 2014 | Kim et al.20 | 18 | Peritoneum | 100% | 10 (55%) | NE | 37 | 50%§ |
| 40%¶ | ||||||||
| 2014 | Saito et al.21 | 16 | Peritoneum | 100% | 13 (81%) | NE | 53 | 53.8%§ |
| 2015 | Fukuchi et al.21 | 40 | Otro | NE | 32 (80%) | NE | 62 | 49%¶ |
| 2015 | Ito et al.23 | 14 | Peritoneum | NE | 14(100%) | 24.8 | 29.5 | 65.6%§ |
| 2015 | Kinoshita et al.24 | 34 | PAN/N3 | 50% | 27 (79%) | NE | NE | 63.5%§ |
| 2017 | Sato et al.25 | 33 | PAN/N3 | 100% | 28 (85%) | NE | 47.9 | 48.6%¶ |
| 2017 | Mieno et al.26 | 31 | PAN/N3 | 77% | 23 (74%) | 53.8 | NE | 71.3%§ |
| 2017 | Uemura et al.27 | 43 | PAN/N3 | 100% | 15 (35%) | NE | 24 | NE |
| 2017 | Einama et al.28 | 10 | PAN/N3 | 100% | 100% | NE | 29 | NE |
| 2017 | Maeda et al.29 | 3 | PAN/N3 | 100% | 3 (100%) | NE | NE | 100%‡ |
| 2017 | Yamaguchi et al.30 | 84 | PAN/N3 | NE | 43 (51%) | 28.5 | 41.3 | NE |
| 2017 | Al-Batran et al. AIO-FLOT331 | 36 | PAN/N3 | NE | 29 (80%) | 27.5 | NE | NE |
| 2018 | Morgagni et al.13 | 33 | PAN/N3 | 91.9% | 22 (67%) | NE | 38 | 39.4%§ |
| 2018 | Beom et al.32 | 101 | PAN/N3 | 75.2% | 57 (56%) | 63.3 | NE | NE |
| 2019 | Solaini et al.33 | 45 | Peritoneum | 91.1% | 30 (67%) | 25 | NE | NE |
| 2019 | Li et al.34 | 81 | PAN/N3 | NE | 66 (81.4%) | NE | NE | NE |
| 2019 | Ramos et al.10 | 16 | T4b | 81.3% | 13 (81.3%) | 8.9 | 11.3 | NE |
| 2019 | Wang et al.35 | 122 | PAN/N3 | 100% | 113 (92.6%) | 63.6 | NE | NE |
| 2019 | Choe et al.36 | 26 | NE | NE | 22 (84.6%) | 36.1 | NE | NE |
| 2019 | Sato et al.12 | 48 | Peritoneum | NE | 35 (72.9%) | 52 | NE | 75.4%§ |
| 2020 | Arigami et al.37 | 13 | Hígado | NE | NE | NE | NE | NE |
| 2020 | Chen et al.38 | 95 | PAN/N3 | NE | 47 (49.5%) | 20.7 | 49.3 | NE |
| 2021 | Yoshida et al.39 | 1902 | PAN/N3 | 79.3% | 839 (69.6%) | NE | 56.6 | NE |
| 2022 | Kano et al.40 | 79 | Peritoneum | 93.7% | 63 (79.7%) | NE | NE | 61.8%§ |
| 2023 | Briceño et al. | 23 | T4b | 87% | 21(91.3%) | 28 | 41.2 | 57.7%§ |
| 38.5% |
*Patients with curative intent surgery (R0) of the total patients undergoing CT. †In all the patients undergoing CT. ‡Overall survival at 2 years. §Overall survival at 3 years. ¶Overall survival at 5 years.
PAN: para-aortic lymph nodes. NS: not specified.
In their paper. Yoshida et al. propose a widely accepted comprehensive classification for CT that focuses on the biology and heterogeneous characteristics of stage IV GC. This classification has helped to establish a common language in this regard5. In these categories the major division is between patients with and without macroscopically detectable peritoneal dissemination. This study focused on patients with locally advanced tumors. with resectable metastases or extra-regional lymph nodes. which can be included in categories 1 and 2. With respect to lymph node involvement. Yoshida establishes a clear difference between involvement of para-aortic lymph node station N° 16a2 (between the celiac artery and the left renal vein) and 16b1 (between the left renal vein and the inferior mesenteric artery). which correspond to category 1. and involvement of other para-aortic lymph nodes. including stations N° 16a1 (aortic hiatus). 16b2 (between the inferior mesenteric artery and the aortic bifurcation) (Fig. 2) and extra-abdominal lymph nodes. which correspond to category 2. However. the CONVO GC-139 study found that survival of patients with para-aortic lymph node metastases in stations N° 16a1/b2 was not inferior to those with metastases in stations N°16a2/b1. This probably supports the growing evidence that tumor biology is similar among cases with hematogenous and lymph node metastases. even with extra-regional involvement. but different from those with peritoneal dissemination.
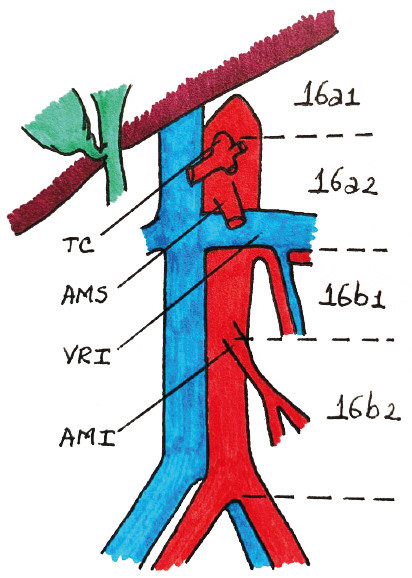
FIGURE 2 Lymph node station N° 16 (paraaoric) and its sub-divisions. TC: celiac artery. AMS: superior mesenteric artery. LRV: left renal vein. AMI: inferior mesenteric artery
Recurrence of GC is considered early if it occurs within two years after curative resection. Numerous studies have investigated predictive factors of recurrence42. It is unclear if this concept is applicable in the CT setting. where a significant percentage of patients experience early recurrence despite R0 resections. Standardization of extended D2+ lymphadenectomy. included in the Japanese Gastric Cancer Treatment Guidelines. 6th edition. after neoadjuvant chemotherapy with extensive lymph node involvement documented on imaging tests prior to systemic therapy. may provide evidence in this regard.
This study has important limitations due to its retrospective nature. the use of multiple treatment schemes in CT. and the fact that it deals with patients from a single center. Further multicenter studies are needed to better characterize this group of patients and their particular aspects in the region.
The ongoing RENAISSANCE and SURGIGAST studies are expected to provide valuable information for optimizing patient selection for CT. standardizing treatment regimens. and better defining the role of surgery in this context.
It is difficult to uniformly categorize and treat all patients with stage IV GC or locally advanced tumors. Since not all cases are eligible for CT. it is necessary to optimize patient selection to maximize efficacy. It is important to consider the risks of this strategy. such as the potential for a decline in postoperative quality of life. limited survival. and early tumor recurrence. We consider that these patients should always be treated by multidisciplinary teams and in reference oncology centers.
In conclusion. CT was an option for rescuing adequately selected patients with advanced GC and returning them to curative treatment. thereby improving the OS rate of this highly lethal condition.
Referencias bibliográficas /References
1. Global Cancer Statistics 2020: GLOBOCAN. 2020. [cited 2022 Dec 16]. Available from: https://gco.iarc.fr/today/online-analysis-multi-bars. [ Links ]
2. Cowling J, Gorman B, Riaz A, Bundred JR, Kamarajah SK, Evans RPT, et al. Peri-operative Outcomes and Survival Following Palliative Gastrectomy for Gastric Cancer: a Systematic Review and Meta-analysis. J Gastrointest Cancer. 2021;52:41-56. [ Links ]
3. Sun J, Song Y, Wang Z, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer. 2013;13:577. [ Links ]
4. Kinoshita J, Yamaguchi T, Moriyama H, Fushida S. Current status of conversion surgery for stage IV gastric cancer. Surg Today. 2021;51:1736-54. [ Links ]
5. Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743-7. [ Links ]
6. Zurleni T, Gjoni E, Altomare M, Rausei S. Conversion surgery for gastric cancer patients: A review. World J Gastrointest Oncol. 2018;10:398-409. [ Links ]
7. Yamaguchi K, Yoshida K, Tanaka Y, Matsuhashi N, Tanahashi T, Takahashi T. Conversion therapy for stage IV gastric cancer-the present and future. Transl Gastroenterol Hepatol. 2016;1:50. [ Links ]
8. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-55. [ Links ]
9. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13. [ Links ]
10. Ramos MFKP, Pereira MA, Charruf AZ, Dias AR, Castria TB, Barchi LC, et al. Conversion therapy for gastric cancer: expanding the treatment possibilities. Arq Bras Cir Dig. 2019;32:e1435. [ Links ]
11. Gallardo-Rincón D, Oñate-Ocaña LF, Calderillo-Ruiz G. Neoadjuvant chemotherapy with P-ELF (cisplatin. etoposide. leucovorin. 5-fluorouracil) followed by radical resection in patients with initially unresectable gastric adenocarcinoma: a phase II study. Ann Surg Oncol. 2000;7:45-50. [ Links ]
12. Sato S, Kunisaki C, Tanaka Y, Sato K, Miyamoto H, Yukawa N, et al. Curative-Intent Surgery for Stage IV Advanced Gastric Cancer: Who Can Undergo Surgery and What Are the Prognostic Factors for Long-Term Survival? Ann Surg Oncol. 2019;26:4452-63. [ Links ]
13. Morgagni P, Solaini L, Framarini M, Vittimberga G, Gardini A, Tringali D, et al. Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg. 2018;53:360-5. [ Links ]
14. Nakajima T, Ota K, Ishihara S, Oyama S, Nishi M, Ohashi Y, et al. Combined intensive chemotherapy and radical surgery for incurable gastric cancer. Ann Surg Oncol. 1997;4:203-8. [ Links ]
15. Yano M, Shiozaki H, Inoue M, Tamura S, Doki Y, Yasuda T, et al. Neoadjuvant chemotherapy followed by salvage surgery: effect on survival of patients with primary noncurative gastric cancer. World J Surg. 2002;26:1155-9. [ Links ]
16. Suzuki T, Tanabe K, Taomoto J, Yamamoto H, Tokumoto N, Yoshida K, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett. 2010;1:743-7. [ Links ]
17. Satoh S, Okabe H, Teramukai S, Hasegawa S, Ozaki N, Ueda S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer. 2012;15: 61-9. [ Links ]
18. Kanda T, Yajima K, Kosugi S, Ishikawa T, Ajioka Y, Hatakeyama K. Gastrectomy as a secondary surgery for stage IV gastric cancer patients who underwent S-1-based chemotherapy: a multi-institute retrospective study. Gastric Cancer. 2012;15:235-44. [ Links ]
19. Han DS, Suh YS, Kong SH, Lee HJ, Im SA, Bang YJ, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol. 2013;107:511-6. [ Links ]
20. Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. 2014;14:266-70. [ Links ]
21. Saito M, Kiyozaki H, Takata O, Suzuki K, Rikiyama T. Treatment of stage IV gastric cancer with induction chemotherapy using S-1 and cisplatin followed by curative resection in selected patients. World J Surg Oncol. 2014;12:406. [ Links ]
22. Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. 2015; 22:3618-24. [ Links ]
23. Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, et al. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res. 2015;35:401-6. [ Links ]
24. Kinoshita J, Fushida S, Tsukada T, Oyama K, Okamoto K, Makino I, et al. Efficacy of conversion gastrectomy following docetaxel. cisplatin. and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41:1354-60. [ Links ]
25. Sato Y, Ohnuma H, Nobuoka T, Hirakawa M, Sagawa T, Fujikawa K, et al. Conversion therapy for inoperable advanced gastric cancer patients by docetaxel. cisplatin. and S-1 (DCS) chemotherapy: a multi-institutional retrospective study. Gastric Cancer. 2017;20:517-26. [ Links ]
26. Mieno H, Yamashita K, Hosoda K, Moriya H, Higuchi K, Azuma M, et al. Conversion surgery after combination chemotherapy of docetaxel. cisplatin and S-1 (DCS) for far-advanced gastric cancer. Surgery Today. 2017;47:1249-58. [ Links ]
27. Uemura N, Kikuchi S, Sato Y, Ohnuma H, Okamoto K, Miyamoto H, et al. A phase II study of modified docetaxel. cisplatin. and S-1 (mDCS) chemotherapy for unresectable advanced gastric cancer. Cancer Chemotherap Pharmacol. 2017; 80:707-13. [ Links ]
28. Einama T, Abe H, Shichi S, Matsui H, Kanazawa R, Shibuya K, et al. Long-term survival and prognosis associated with conversion surgery in patients with metastatic gastric cancer. Mol Clin Oncol. 2017;6:163-6. [ Links ]
29. Maeda O, Matsuoka A, Miyahara R, Funasaka K, Hirooka Y, Fukaya M, et al. Modified docetaxel. cisplatin and capecitabine for stage IV gastric cancer in Japanese patients: a feasibility study. World J Gastroenterol. 2017;23:1090-7. [ Links ]
30. Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. 2018;21:315-23. [ Links ]
31. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIOFLOT3 Trial. JAMA Oncol. 2017;3:1237-44. [ Links ]
32. Beom SH, Choi YY, Baek SE, Li SX, Lim JS, Son T, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer. 2018;18:1116. [ Links ]
33. Solaini L, Ministrini S, Bencivenga M, D'Ignazio A, Marino E, Cipollari C, et al. Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer. 2019;22:1285-93. [ Links ]
34. Li W, Jiang H, Yu Y, Wang Y, Wang Z, Cui Y, et al. Outcomes of gastrectomy following upfront chemotherapy in advanced gastric cancer patients with a single noncurable factor: a cohort study. Cancer Manage Res. 2019;11:2007-13. [ Links ]
35. Wang T, Wang N, Ren H, Zhou H, Zhou A, Jin J, et al. Long-term Results of Conversion Therapy for Initially Unresectable Gastric Cancer: Analysis of 122 Patients at the National Cancer Center in China. J Cancer. 2019;10:5975-85. [ Links ]
36. Choe HJ, Kim JW, Han SH, Lee JH, Ahn SH, Park DJ, et al. Conversion Surgery in Metastatic Gastric Cancer and Cancer Dormancy as a Prognostic Biomarker. Cancers (Basel). 2019;12:86. [ Links ]
37. Arigami T, Matsushita D, Okubo K, Kawasaki Y, Iino S, Sasaki K, et al. Indication and Prognostic Significance of Conversion Surgery in Patients with Liver Metastasis from Gastric Cancer. Oncology. 2020;98:273-9. [ Links ]
38. Chen GM, Yuan SQ, Nie RC, Luo TQ, Jiang KM, Liang CC, et al. Surgical Outcome and Long-Term Survival of Conversion Surgery for Advanced Gastric Cancer. Ann Surg Oncol. 2020;27:4250-60. [ Links ]
39. Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, et al; CONVO-GC-1 Study Group. Federation of Asian Clinical Oncology (FACO). International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. 2021;6:227-40. [ Links ]
40. Kano Y, Ichikawa H, Hanyu T, Muneoka Y, Ishikawa T, Aizawa M, et al. Conversion surgery for stage IV gastric cancer: a multicenter retrospective study. BMC Surg. 2022;22:428. [ Links ]
41. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. 2016; 19:329-38. [ Links ]
42. Jiao X, Wang Y, Wang F, Wang X. Recurrence pattern and its predictors for advanced gastric cancer after total gastrectomy. Medicine 2020; 99:51(e23795). [ Links ]
Received: August 10, 2023; Accepted: February 05, 2024











 texto em
texto em