Introduction
In oviparous reptiles, ovarian recrudescence and vitellogenesis are influenced by numerous environmental, social, and hormonal factors. In this way, climatic factors, such as temperature, photoperiod, and rainfall; and social factors, such as the sexual composition of the group and the attractiveness of the male, considerably influence ovarian follicular recruitment (Jones, 2011; Santos et al., 2015).
Male vertebrates use sensory signals (chemical, visual, auditory, and / or tactile) to activate specific neuroendocrine responses in the female. Some of these signals regulate the release of gametes, as in the case of spawning in some fishes and induced ovulation in mammals, such as cats, ferrets, and rabbits (Chaffaux, 1993). This phenomenon has also been reported in reptiles such as the loggerhead sea turtle, Caretta caretta (Manire et al., 2008). Other signals affect gamete maturation; for example, in Python curtus (DeNardo and Autumn, 2001) and Vipera aspis snakes, copulation is an obligatory stimulus for the initiation of vitellogenesis, presumably to guarantee that the females mobilize organic reserves only in the presence of the male, and thus, ensure fertilization. In Thamnophis sirtalis parietalis, copulation plays a critical role for vitellogenesis; however, in some reproductive seasons, unmated females can also become vitellogenic (Jones, 2011).
A wide variety of sexual behaviors have been described in single-sex groups in different taxa of vertebrates and invertebrates, such as mammals, birds, reptiles, amphibians, insects, mollusks, and nematodes (Bailey and Zuk, 2009; Scharf and Martin, 2013). In squamata reptiles, sexual behaviors with members of the same sex have been described. In the parthenogenic lizard Cnemidophorus uniparens, for example, gonadal recrudescence is stimulated by females with pseudo-male behaviors (Crews et al., 1986). This evidence supports the hypothesis that, in S. merianae, as in other species, vitellogenesis can occur independently of the presence of the male (DeNardo and Autumn, 2001).
In Salvator merianae the effects of copulation on follicular dynamics are still not entirely clear. Manes et al. (2007) proposed that copulation is a necessary stimulus for vitellogenesis to occur. They also proposed that follicular atresia in the absence of copulation could represent a timely exit from the follicular cycle, which would avoid the great energy effort involved in vitellogenesis. However, in later studies in S. merianae, García- Valdez et al. (2011) suggest that the effect of mating or the sole presence of the male is more related to egg laying than to yolk synthesis.
The aims of this work were to: a) evaluate the influence of sexual interactions on ovarian cycle development; b) analyze the behavior of females in the presence of males and in different conditions of sexual isolation.
Materials and methods
Animals and study conditions
The studies were carried out with animals from the Experimental Lizard Hatchery of Facultad de Agronomía y Zootecnia of Universidad Nacional de Tucumán, northwest of Argentina (26º51’S and 65º17’W). The snout-vent lenght of the evaluated adult females averaged 39.7 ± 0.48 cm, and the average weight was 3.38 ± 0.11 kg. Besides their adult size, selected females had oviposited at least once. The animals were kept in open-air enclosures with 1.2 m tall masonry walls, provided with shelters with dry grass, shaded spaces and ad libitum water. In all cases the minimum area of 2 m2 suggested per animal was considered (Manes, 2016). To enable individual monitoring, an 11.5 x 2.1 mm subcutaneous microtransponder (ID- 100A, TROVAN Electronic Identification Devices LTD, Rosenbusch, Buenos Aires) was implanted into the left abdominal flank.
The animals were fed ad libitum with a ration specially designed for the hatchery, consisting of 85 % ground chicken heads and feet (1:2), 15 % soy flour, 0.25 % vitamin and mineral supplement (Micromix, Biofarma, Córdoba, Argentina),
0.25 % sodium chloride, and 0.1 % butyl hydroxy toluene (Vega-Parry and Manes, 2000).
Experimental design
Thirty-five Salvator merianae females were monitored over five years: 14 individuals were monitored during one reproductive cycle, 11 during two cycles, and 11 during three cycles, of a total of 69 analyzed cases. Four experimental groups were formed: Females in presence of males (in the hatchery): A- Females with males (n = 30): groups made up of 4-5 females and one male (reproductive family). B- Isolated females in hatchery: group consisting only of females (n = 11), in enclosure adjacent to groups with males. Females in absence of males: C- Sexually isolated females in a group (n = 13): four or five females sharing an enclosure. D- Females in individual sexual isolation (n = 15): females in individual enclosures.
The females with males were kept in the Experimental Lizard Hatchery of the Facultad de Agronomía y Zootecnia of Universidad Nacional de Tucumán, Argentina. The females without males were kept in facilities with the same structural characteristics, more than 5 km away from the hatchery, to avoid the influence of chemical (pheromones), auditory or other signals emitted by the male. For each reproductive season, new groups were formed, by means of a random redistribution of the animals. Females destined for sexual isolation were separated from March (reproductive quiescence period) to the end of December (end of incubation season).
The studies were carried out in five predetermined stages of the annual cycle: 1- hibernation (H) in July; 2- hibernation emergence (HE) in mid- September; 3- courtship-mating (CM) in October; 4- oviposition (O) in November; and 5- incubation (I) in December. The CM stage corresponded to the period of sexual interactions. Oviposition was considered when more than 70 % of the females of the breeding families of the hatchery had already laid eggs. The studies corresponding to the incubation stage were carried out towards the middle of the incubation period (approximately
30 days after oviposition). The ultrasounds of the females in sexual isolation, corresponding to the stages of CM, O and I, were obtained simultaneously with the reproductive group.
For the ultrasound studies was used a Mindray DP-6600 Vet with a linear transducer model 75L38EA, frequency 5-10 MHz. The images obtained were saved in digital format. To evaluate follicular condition, we followed the criteria suggested by García-Valdez et al. (2011): non- vitellogenic follicles were those smaller than 10 mm in diameter and with totally anechoic content. Those greater than 10-12 mm in diameter with echogenic content were considered vitellogenic. Initial vitellogenesis was to take place considered when follicles had a 10-14 mm diameter with increased peripheral echogenicity, and advanced vitellogenesis was established when follicles were greater than 14 mm in diameter, and sonographically homogeneous (studies not included in this work).
To evaluate the effect of mating as a trigger signal of vitellogenesis, copulation monitoring was carried out in 20 females. The enclosure with reproductive families was observed daily. Copulations were recorded by an observer located outside the enclosure. Immediately after observing the copulation, follicular diameter and echogenicity of copulated females were recorded by ultrasound. To avoid undetected copulations, the males were kept away from the enclosure during the afternoon and evening, until monitoring the following morning.
Statistical analysis
The χ2 test for homogeneity of proportions was used to evaluate the frequencies of females with and without vitellogenic cycle in the defined groups.
The mixed model methodology approach was applied for the analysis of follicular diameters. The experimental group, time of life cycle and the interaction between both effects were included in the model as fixed effects. This approach models repeated measures and lack of independence by including random effects. Year and animals nested each year were considered random variables in this model. In addition, corrections were made for heterocedasticity when necessary. The best-fitting models were selected based on a lower value of the Akaike´s information criterion (AIC) and the significance obtained through the likelihood ratio test, in which alternative models with an equal fixed part and a different random component were compared. Fixed effects were tested by F statistics provided by the Wald tests. The values corresponding to females that underwent vitellogenic cycles and those of females that did not, were analyzed separately with the same structure of fixed and random effects. The main effects were compared by the DGC test (Di Rienzo et al., 2002) for multiple comparisons (non-vitellogenic females) or contrasts between groups at each moment of the cycle (vitellogenic females). This form of comparison was defined in the latter case because the DGC test showed little capacity to detect significant differences at each moment of the cycle. The level of significance (α) was 0.05.
Finally, to calculate average daily growth rates between hibernation emergence and courtship- copula output, a regression model was adjusted with the following explanatory variables: days (numerical variable), condition (classificatory variable) and the interaction between them, with the purpose of determining whether the adjustment lines coincided, were parallel or if it was necessary to run a different setting for each group.
In all cases, the statistical package R (R Core Team, 2021) was used with the graphic interface included in InfoStat (Di Rienzo et al., 2017).
Results
Behavioral studies
During the study, the females of the group A behaved “normally”, and showed no attempts to attack one another, or escape. The females emerged from hibernation a few days after the males, and around 15-20 days post-emergence, they accepted male courtship. This behavior consisted of chases by the male, with attempts to restrain the female by biting her by the tail and attempts to mount her. Copulations were recorded by an observer located outside the enclosure (supplementary material). Of the 20 monitored females, 16 were receptive to the male and mating in the following days. Each of these females copulated once to five times per season.
In the three groups of females without males, no sexual behaviors were observed among them. The females of group B were calm, and did not present unusual behaviors.
The females in group C were more aggressive (one of them even had to be removed due to severe injuries caused by fights). Also in this group, numerous attempts to escape were observed, mainly in the months of greatest reproductive activity (from October to December). Females of group D showed an intermediate aggressive behavior between groups A and C, with few attempts to escape.
Vitellogenic cycles in the different experimental groups
The chi-square test for homogeneity of proportions reflected that the proportions of vitellogenic females were different in the different experimental conditions. In the reproductive group A, the occurrence of vitellogenic cycles (24/30) was higher than in the 3 groups with sexual isolation.
As for sexually isolated groups, in group B, of the 11 females isolated in the hatchery, 7 showed vitellogenic cycles. Of the females in group C (in- group isolated), 4 of 13 underwent vitellogenesis. In group D (females isolated individually), 7 out of 15 animals presented vitellogenic cycles.
The lowest incidence of vitellogenesis was observed in in-group sexually isolated females, which presented less than half of the vitellogenic cycles that females in reproductive families underwent. The percentages of vitellogenic and non-vitellogenic cycles for each experimental condition are shown in Table 1.
Table 1 Percentages of vitellogenic cycles for each experimental group of Salvator merianae females.
| Experimental condition | Number of females | Vitellogenic cycles | Non-vitellogenic cycles |
|---|---|---|---|
| Females with males1 (Group A) | 30 | 80 % | 20 % |
| Isolated females in hatchery1 (Group B) | 11 | 64 % | 36 % |
| Sexually isolated females in a group2 (Group C) | 13* | 31 % | 69 % |
| Individual sexual isolation 2 (Grup D) | 15 | 47 % | 53 % |
1Females in the hatchery. 2Females in enclosures without males. *Of the 15 original females for this group, one was separated due to severe injuries and another escaped. χ2 = 10.86, d.f. = 3, p-value = 0.0125.
The follicular diameters of the different experimental groups were recorded with ultrasound throughout the annual ovarian cycle (Figure 1). Of the females that underwent vitellogenic cycles, the females of group A presented a significantly greater follicular size during the period of sexual activity (courtship-mating, oviposition, and incubation) compared to the 3 conditions of sexual isolation. Of the 24 vitellogenic females in the reproductive group, 14 oviposited. The remaining 10 females continued follicular growth without oviposition. In the 4 groups, a decrease in follicular size was observed in the incubation stage (December), corresponding to the onset of atresia of non-ovulated follicles.

Figure 1 Follicular diameters of the females that underwent vitellogenic cycles in the 4 experimental groups (H: hiber- nation; HE: hibernation emergence; CM: courtship-mating; O: oviposition, I: incubation). Different letters in the vertical direction denote significant differences through contrasts with 1df (p < 0.05). Mean values ± standard error. Black circle: reproductive group; black box: isolated in hatchery; gray box: females in individual isolation; white box: females in group isolation.
In females that underwent non-vitellogenic cycles, follicular diameter varied according to the stage of the annual cycle, but no differences were observed among the different experimental conditions (experimental condition: df = 3, 18, F = 0.81, p-value = 0.5025; Stage: F = 42.81, df = 4, 80, p-value <0.0001; condition x stage: F = 1.69, df = 12, 80, p-value = 0.0851, AIC = 497.17). The mean follicular diameters calculated in females that underwent non-vitellogenic cycles were 3.20 ± 0.56 mm in hibernation, 4.42 ± 0.62 mm in hibernation emergence, 7.26 ± 0.65 mm in courtship-mating, 7.46 ± 0.62 mm in oviposition and 4.58 ± 0.80 mm in incubation (Figure 2).
Follicular growth rates were compared among the females that underwent vitellogenic cycles in the 4 experimental conditions. The analysis using mixed models reflected a significant interaction between experimental condition and follicular growth time (condition x days: F = 2.91, df = 3, 142, p-value = 0.0367, AIC= 935.93), and growth rate was determined for each experimental condition. The growth rate of the reproductive group was significantly higher than the rate calculated for the 3 conditions of sexual isolation. Daily follicular growth from hibernation emergence to oviposition, was: for group A, 0.34a ± 0.03 mm / day; for group B, 0.20b ± 0.05 mm / day; for group C, 0.26b ± 0.04 mm / day; and for group D, 0.22b ± 0.03 mm / day.
No significant differences were found in ovarian follicle growth rate among the females under different experimental conditions that underwent non-vitellogenic cycles (F = 0.49, df = 3, 9, p-value = 0.6898, AIC = 447.74).Therefore, a single growth rate of 0.04 ± 0.01 mm/day was calculated.
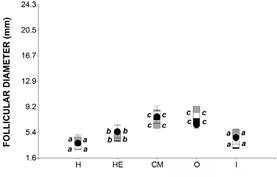
Figure 2 Follicular diameters of the females that underwent non-vitellogenic cycles in the 4 experimental groups (H: hi- bernation; HE: hibernation emergence; CM: courtship-ma- ting; O: oviposition, I: incubation). Different letters denote significant differences according to the DGC Comparison Test (p < 0.05). Mean values ± standard error. Black circle: reproductive group, black box: isolated in hatchery, gray box: females in individual isolation, white box: females in group isolation.
Copulation-vitellogenesis relationship
Of the total number of group A females monitored during sexual interactions, 80 % were receptive to the male. Copulations occurred between the second and fourth week after finishing hibernation. The ultrasound monitoring after copulation showed that 87.5 % (n = 14) of the females had vitellogenic follicles, and only 2 females (12.5 %) were in the previtellogenic stage. In the case of the females that rejected the male, only one stopped its follicular growth in the previtellogenic stage, while the other three underwent anovulatory vitellogenic cycles.
An inverse relationship was observed between the degree of follicular development at the time of copulation and the copulation-oviposition interval (r = -0.88, p-value = 0.0008). The larger the size and echogenicity of the ovarian follicles were at the time of mating, the shorter the copulation- oviposition interval was. Females that copulated with fully developed vitellogenic follicles had a mean interval between copulation and oviposition of 23.4 days. Instead, of the 2 females that copulated with previtellogenic follicles, only one of them oviposited, with its mating-oviposition period of 44 days being notably longer than that of the rest of the females.
Table 2 shows the size and ultrasound appearance of the ovarian follicles of the females from the reproductive group at the time of mating, and their copulation-oviposition interval.
Table 2 Ovarian follicular condition of receptive females of Salvator merianae at the time of mating (mean ± standard error).
| Oviposition | N° | Follicular condition at mating | Average follicular diameter at mating (mm) | Mating-oviposition interval (days) |
|---|---|---|---|---|
| With egg laying | 1 | Previtellogenic | 6.94 ± 0.65 | 44 |
| (n = 10) | 3 | Initial vitellogenesis | 12.73 ± 0.94 | 29 ± 4.7 |
| 6 | Advanced vitellogenesis | 19.33 ± 1.85 | 18 ± 4.9 | |
| No egg laying | 1 | Previtellogenic | 7.30 ± 0.19 | - |
| (n = 6) | 2 | Initial vitellogenesis | 13.90 ± 1.1 | - |
| 3 | Advanced vitellogenesis | 15.15 ± 0.05 | - |
Discussion and conclusions
In oviparous reptiles, environmental, nutritional, and social factors strongly influence ovarian follicular development and vitellogenesis (Jones, 2011). In South American lizard Salvator merianae the effect of copulation on vitellogenesis and ovulation is still debated. Previous observations in
S. merianae proposed copulation as a triggering stimulus for vitellogenesis and subsequent ovulation (Manes et al., 2007). However, later studies described vitellogenic cycles in females that did not copulate and suggested that the effect of mating or the presence of the male would be more related to egg laying than to yolk synthesis (García-Valdez et al., 2011). Induced ovulation is frequent among eutherian mammals, however, there are few reports among reptiles, such as Caretta caretta sea turtles (Manire et al., 2008). In this work we demonstrate that vitellogenesis in captive S. merianae can occur both, in the presence and in the absence of male individuals.
The females of group A presented a higher proportion of vitellogenic cycles, a higher follicular growth rate, and a greater ovarian follicle development with respect to the females in the 3 conditions of sexual isolation. During the reproductive period S. merianae males display a rich diversity of behaviors, including aggressive behaviors, territorial marking, persecutions of females, with bites applied to the tail and neck, panting, mounting, and scratching on the side by the male, with its hind legs (Noriega et al., 1996). These courtship behaviors probably generate a positive response in the female’s ovarian recrudescence and/or vitellogenesis.
Although the presence of the male was not essential for the occurrence of vitellogenesis, females in contact or near the male showed a higher proportion of vitellogenic cycles and reached greater follicular development. Among the reproductive behaviors displayed by males, during territorial marking male individuals typically rub their thighs and cloacal region on the ground (Mercolli and Yanosky, 1989), dispersing the product of the femoral glands, apparently involved in the production of semiochemical signals (Chamut et al., 2009). Possibly these chemical signals that the males disperse in the environment can be captured by the females from nearby pens, and these generate a stimulating effect. Recent studies (Richard et al., 2020) have shown that female S. merianae exhibited stronger conspecific trailing abilities, showing a high response to sex- specific odor of males. This is probably related to the important development of the vomeronasal system documented for these lizards (Baxi et al., 2006; Sánchez Loria et al., 2013).
Regarding the influence of copulation on vitellogenesis, the finding of vitellogenic cycles in females in sexual isolation, added to the fact that the copulations monitored in the reproductive group occurred with already vitellogenic follicles, would indicate that the beginning of vitellogenesis is independent of mating.
An indirect relationship between follicular development at the time of copulation and the copulation-oviposition interval was determined. According to our observations, the only female that copulated in previthellogenesis and laid eggs had a copulation-oviposition interval 20 days longer than the average of those that copulated in vitellogenesis. This shows that female S. merianae can reserve viable sperm for at least 44 days. This ability of females to reserve sperm has been described in various groups of reptiles (Sever and Hamlett, 2002). Possibly, this ability allows the female to ensure fertilization during a period that would lead to the young offspring being born under favorable environmental conditions to feed before the first hibernation (DeNardo and Autumn, 2001).
It has been shown that stressors in animals produce alterations in their reproductive behavior and physiology (Tokarz et al., 2011). Among the alterations of sexual behavior, same-sex sexual behaviors (SSB) have been observed in species of mammals, birds, reptiles, amphibians, insects, mollusks and nematodes (Bailey and Zuk, 2009). These SSB can be promoted by severe partner shortage conditions (Bonnet et al., 2016). In our study we did not observe SSB among females isolated from males. Regarding reproductive physiology, the increase in stress-related hormones, such as corticosterone, may affect gonadal function, reproductive behavior, egg laying, and other aspects related to reproduction (Tokarz et al., 2011). This is consistent with our observations that S. merianae females, sexually isolated in groups, exhibited the highest levels of aggressiveness and the lowest proportion of vitellogenesis of all groups studied. This suggests an inhibitory effect (not suppressive) on vitellogenesis, possibly due to stress.
In this work we provide useful data for the knowledge of the reproductive cycle and the bases of sexual behavior of S. merianae females in captivity. Understanding the reproductive physiology of the Salvator female and the regulatory factors of vitellogenesis is essential in order to achieve efficient reproductive, nutritional and health management. In the same way, these contributions will be useful for designing and developing of control tools for these lizards in regions where they are invasive.















