Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO  uBio
uBio
Compartilhar
Ecología austral
versão On-line ISSN 1667-782X
Ecol. austral vol.29 no.2 Córdoba ago. 2019
ARTICLE
Effect of water increment on phenology, productivity, and herbivory of Fuchsia boliviana C. (Onagraceae) in Northwestern Argentina
Silvia B. Lomáscolo*; A. Carolina Monmany-Garzia; Julieta Magro; Pablo Suárez y Franco Andrada
Instituto de Ecología Regional (IER), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) - Universidad Nacional de Tucumán (UNT).
ABSTRACT
Water availability may affect plant productivity because it limits a plant's ability to reproduce and defend itself. In this field study, we studied the effect of water increment in relation to: a) flower production, b) fruit production and c) herbivory, focusing on Fuchsia boliviana (Onagraceae) in the Yungas forest of Northwestern Argentina. We selected 10 pairs of plants of similar size separated at least 5 m from each other. We watered one plant of the pair with 20 L twice a week between September and December, 2016. The number of flowers and fruits of all plants were counted twice a week. Herbivory was quantified once from photographs of five leaves per plant using ImageJ version 1.5i. Based on Wilcoxon tests, we found no difference in the number of flowers, or fruits, nor in herbivory in plants of both treatments. We observed a trend for watered plants starting to flower later and with more intensity than unwatered plants. We discuss different options that may explain why water increase did not affect reproduction nor the level of herbivore damage in Fuchsia boliviana.Keywords: Fruit production; Flower production; Herbivory; Water regime; Climate change; Yungas; Argentina.
RESUMEN
Efecto del incremento de agua en la fenologí, productividad y herbivorí de Fuchsia boliviana C. (Onagraceae) en el noroeste argentinoLa disponibilidad de agua puede afectar la productividad de una planta ya que condiciona su habilidad para reproducirse o defenderse. Examinamos a campo el efecto del aumento de agua sobre a) la producción de flores, b) la producción de frutos y c) la herbivorí de Fuchsia boliviana (Onagraceae) en el bosque de Yungas del noroeste argentino. Seleccionamos 10 pares plantas de tamaño similar, separadas al menos 5 m entre sí, y regamos una planta del par con 20 L de agua dos veces por semana entre septiembre y diciembre de 2016. Contamos el número de flores y frutos de todas las plantas dos veces por semana. Cuantificamos la herbivorí una sola vez en cinco hojas por planta en base a fotografís. No encontramos diferencias en el número de flores o frutos ni en la herbivorí entre plantas de ambos tratamientos. Observamos una tendencia a que las plantas regadas comenzaran a florecer más tarde y con más intensidad que las plantas no regadas. Se discuten algunas posibilidades para explicar por qué el incremento de agua no afectó la reproducción ni la tasa de daño en Fuchsia boliviana.
Palabras clave: Producción de frutos; Producción de flores; Régimen de agua; Cambio climático; Yungas; Argentina.
Recibido: 21 de Agosto de 2018
Aceptado: 11 de enero de 2019
Editor asociado: Alejandro Farji-Brener
INTRODUCTION
Water availability is one of the most important determinants of productivity in terrestrial environments as it may affect different aspects of plant fitness, such as flower and fruit production and defense of photosynthetic and reproductive tissues (Bernacchi and Vanloocke 2015). A first aspect of fitness are flower production and pollination. It has been shown that flowers represent a key water cost to plants (Lambrecht 2013) as flower stomata do not close as do leaf stomata (Galen 2005; Dudley et al. 2018). Therefore, flower production and maintenance is often determined by water availability (Dudley et al. 2018). Water is involved in several processes that lead to proper functioning of the flower as an attractant to pollinators, such as turgor maintenance and nectar production and concentration, as well as in the maintenance and proper functioning of the reproductive organs themselves (Galen 2005 and references therein). A second aspect of plant fitness is fruit production and dispersal of seeds. Fruit production may also be affected by a change in water regime indirectly by a modification of the number or longevity of flowers (Eckhart et al. 2010; Dudley et al. 2018), or of pollinator attractiveness via altered floral rewards or flowering phenology (Carroll et al. 2001; Galen 2005). A decrease in attractiveness may, in turn, reduce pollinator visits and, concomitantly, the number of fruits produced. More directly, fleshy fruits contain abundant water and it has been shown that, although plants have physiological strategies to counteract a decrease in water availability, drought leads to fruit abortion (Case and Ashman 2005; Zanor et al. 2009). Yet there is also evidence that a plant´s capacity to flower and fruit may be diminished with increased rainfall, probably due to decreased pollinator activity or increased risk of pathogen infection (ChangYang et al. 2016). Thirdly, defense against herbivores is another important aspect of plant fitness. There is some evidence showing that plants defend themselves better when water is added experimentally to Asclepias syriaca (Apocynaceae) (Hahn and Maron 2018) and to the sagebrush, Artemisia tridentate (Asteraceae) (Pezzola et al. 2017). Yet the direction of the effect of water change on a plant’s ability to defend itself might not be straightforward because for some species water limitation may stimulate the production of secondary metabolites, which could potentially increase plant defenses (Ripoll et al. 2014). In sum, water availability is a key factor that directly and indirectly determines plant fitness.
Subtropical rainforests are seasonal, with a marked dry season during the months before and at the beginning of flowering of most species. In this scenario, we would expect that incrementing water just before the wet season starts will affect flowering, fruiting and defense capacity of native plants in this environment. In this field study we examined how water increment influenced a) flower production, b) fruit production and c) herbivory, focusing on an understudied species of the Yungas forest in northwestern Tucumán,Fuchsia boliviana Carrière (Onagraceae). We selected this species because, although we found no information on its overall biology involving water dependency, soil demand, fruiting or flowering capacity, or root anatomy in relation to water absorption, it shows two characteristics that, we think, make it a good candidate to understand the response to changes in water regime: a) it grows in disturbed environments next to river banks (Berry 1982) or intermittent water courses and, therefore, is presumably highly affected by water availability, and 2) it grows away from the microclimatic protection of the forest, on sandy soils, in environments that are exposed to direct solar radiation and concomitant rapid loss of soil water. Therefore, the humidity content of the microsites where F. boliviana is found may be subjected to important variation. We predicted that the addition of water, starting in the dry season and continuing throughout the complete flowering and fruiting season, would affect the herbivory level and the production of flowers and fruits of F. boliviana.
MATERIALS AND METHODS
Study site
he study site was located in the Sierra de San Javier Provincial Park, Tucumán, Argentina (26°43´00"S - 65°22´00"O). The park has an area of 14000 ha of Yungas forest, which is the southernmost extension of the Andean montane forests (Cabrera and Willink 1980). The climate is subtropical, with a marked seasonality; the dry season occurs from May to September, and the rainy season occurs from November to March, with intermediate climatic conditions in April and October (Figure S1, Table S1). Mean annual precipitation varies between 1300 and 1500 mm, and mean annual temperature is 18 °C (Hunzinger 1997), with a marked dry season in the winter, between June and October (Supplementary Material). The soils in the area have been classified as Inceptisol soils, which are immature and characterized by being rich in organic matter (Puchulu and Fernández 2014). Vegetation corresponds to the intermediate Yungas' altitudinal level known as Montane forest, a semideciduous forest distributed between 700 and 1000 m. a. s. l., which, in comparison with the three altitudinal levels of the Yungas forest, receives the most abundant precipitations. The forest canopy is dominated by Ocotea porphyria (Lauraceae), Blepharocalyx salicifolius (Myrtaceae), Pisonia zapallo (Nyctaginaceae), and Parapitadenia excelsa (Fabaceae); while common understory trees are Piper tucumanum (Piperaceae), Eugenia uniflora (Myrtaceae), Myrcianthes pungens (Myrtaceae), Allophyllusedulis (Sapindaceae), and Urera caracasana (Urticaceae) (Grau 2002; Malizia and Grau 2006; Malizia et al. 2013).
The specific site where we conducted the experiment was the bed of an intermittent creek with a slope of 4.6° (measured at five random spots and averaged). The soil there was rocky and sandy, and the plants were typical disturbance colonizers, such as Solanum riparium(Solanaceae), Piper hieronymi, Boehmeria caudata, Tecoma stans, Morus alba, Morus nigraand many herbaceous species.
Study species
Fuchsia sp. is a genus of the Onagraceae family that includes about 110 species of tropical and subtropical distribution in America and Oceania (Berry et al. 2004). The majority of species is distributed in South America; some are in Central America and Mexico, and some in New Zealand and Tahiti. Fuchsia sp. is a mesophytic genus; that is, it grows in soils of moderate humidity and relatively humid atmosphere and avoids soils with standing water (Carlquist 1975; Leathwick and Whitehead 2001). Species within the genus Fuchsia, which are shade-tolerant, often form roots when light levels increase (Caranqui Aldaz 2011), though there are some examples of Fuchsia sp. responding more to temperature than to light exposure (Graves and Zhang 1996). Some exceptional cases have been observed where Fuchsia (pachyrrhiza) form tuberous roots storing starch in accordance with a strongly seasonal habitat and a deciduous habit of the species (Berry et al. 1988). The genus comprises animal-pollinated species, mainly by nectarivorous birds. Most species of the genus are monoecious, while nine species show sexual dimorphism (Berry et al. 2004).
We studied Fuchsia boliviana Carrière, a shrubby species of about 3.5 m in height that grows in the Austral Yungas in NW Argentina. The species is usually found in the rocky and sandy soils of humid slopes, frequently on river and stream banks. Studies conducted on the wood anatomy of the species suggest that F. boliviana lacks some anatomical features related to marked seasonality in other Onagraceae, such as interxylary phloem (Carlquist 1975). Information is largely lacking for this species about root formation or how far down roots grow in response to water availability, and the information for other Onagraceae (Hussner 2010) and other Fuchsia evidence high plasticity in terms of root development. Fuchsia boliviana reproduces vegetatively though the mechanism would take place through trailing stems or broken stems that root (Berry 1982). In consequence, one main stem per individual is easily identified. Stem ramifications are observed near the ground, but, in most cases, ramifications are higher. The whorled leaves are around 15 cm-long, with entire margin and long petiole. Low levels of herbivory have been observed in the field, in comparison with other species in the area (Monmany-Garzia and Lomáscolo, personal observation). Trichomes and secondary compounds are present in the species (Breedlove et al. 1982; Averett et al. 1986), though we found no previous study on herbivory related to the species. The 6 cmlong flowers are pendant, tubular, red, and are disposed in corymbs; the main pollinators are hummingbirds (J. Magro, personal observation). The fruits are small berries measuring 1.7 cm long and 1 cm wide. When mature, the fruits are dark violet and they are dispersed by birds (Giannini 1999). Each fruit contains on average 220.5 small seeds of 0.3 g each (J. Magro and P. Blendinger, personal observation).
Fieldwork
We selected and measured 20 individuals of F. boliviana that were paired according to overall plant size (height and trunk diameter at the base) and physical proximity. The plants included in this study ranged between 1.5 and 4 m in height. We made sure that plants were no closer than 5 m apart so that watering one plant did not affect any other plants in our study. Within each of the 10 pairs we randomly assigned the treatment, water vs no-water (see below), using a coin. The effect of water increment was determined by watering the individuals of each pair assigned to the "water" treatment with 20 L of water from a nearby stream twice a week. The other individual of the pair, in the "unwatered" treatment, was not watered; instead, it was left under natural precipitation conditions. According to the precipitation data for the rainy season collected by the meteorological station run by our institute in Horco Molle, 20 L of water twice a week (equivalent to 5.7 mm/day) would be close to a 60% increment with respect to regular water received from natural precipitation (3.6 mm/day). For flower and fruit analysis one of the pairs was left out because they had very large numbers of fruits and flowers, and fell well outside the range of the other plants. Therefore, we considered them outliers. Results were qualitatively similar with or without those plants so we report here only results from analyses without the outliers.
When plants started flowering, by October 7 (Figure 1), we started counting the flowers of each individual each time we went to the field to water the plants. For data analysis we considered flowers that were open or about to open, that is, when full-sized and the tip seemed a bit bloated, as this was an indicator that the flower was ready to open within a few hours (Lomáscolo and MonmanyGarzia, personal observation). By November 2, plants started to show ripe fruits. The fruits considered in the analysis were those that showed signs of maturity, i.e., had already turned from bright red to dark violet (Figure 1). To compare the number of flowers and fruits produced by plants of each treatment, we used the mean number of flowers/fruits calculated over all visits to the plants and across plants of each treatment. We did not use the total number of flowers/fruits because we would have needed to identify each flower/ fruit, and marking them individually was too difficult because of the high number of flowers/fruits produced by many of the plants. Using the mean number seemed to represent reasonably well the number of flowers/fruits produced, as averaging plants from each treatment per visit gave a smooth pattern with a logical production peak in the middle of the flowering/fruiting season (Figure 2). Moreover, the number of flowers/fruits produced by each plant and, concomitantly, the overall production of all the plants in the study, did not fluctuate much between visits, and therefore calculating an average seemed to represent well the production of flowers/ fruits observed in the field.

Figure 1. A) Flowers of Fuchsia boliviana (Onagraceae). We considered for analysis open flowers and those that were just about to open, which had a bloated tip, as signaled by the two arrows. B) Ripe and unripe fruits of the same species. We considered for analysis only those fruits that had completely turned dark violet/black. C) A F. boliviana shrub in its typical habitat in our study site, on the dry sandy/rocky soil of an intermittent creek course. All plants chosen for our treatments were far enough from the water course to avoid natural water when the creek was not dry. D) Watering a small F. boliviana plant right at its base, trying to avoid runoff as much as possible. Watering was done slowly using small 5-L containers so that water had time to be absorbed into the sandy soil.
Figura 1. A) Flores de Fuchsia boliviana (Onagraceae). Para el análisis consideramos las flores abiertas y aquellas que estaban a punto de abrirse, que tenían la punta hinchada, como se señala con las dos flechas blancas. B) Frutos maduros e inmaduros de la misma especie. Consideramos para el análisis sólo aquellos frutos que ya estaban completamente violeta oscuro/negros. C) Un arbusto de F. boliviana en su hábitat típico en el suelo arenoso/rocoso del cauce seco de un arroyo intermitente. Todas las plantas elegidas para los tratamientos estaban lejos del cauce para evitar el riego natural por el agua corriente cuando el arroyo crecía. D) Riego de una planta pequeña de F. boliviana justo en su base, intentando evitar lo mejor posible el escurrimiento del agua. Regamos con recipientes chicos, de 5 L para dar tiempo a que el agua se absorbiera en el piso arenoso.
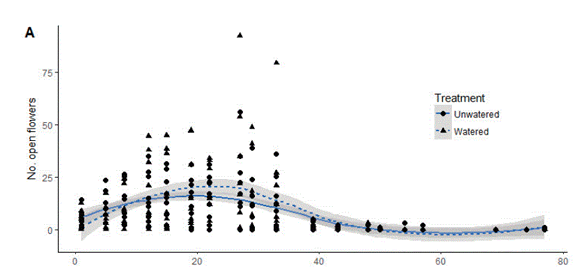
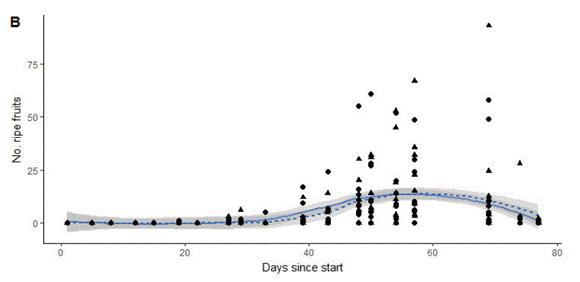
Figure 2. Phenology of Fuchsia boliviana. Plot of the number of open flowers (A) or ripe fruits (B) of Fuchsia Boliviana (Onagraceae) throughout the duration of the study. The date of the first visit was September 15, and the last day was December 22. For each visit, we plotted watered (triangle) and unwatered plants (circle).
Figura 2. Fenología de Fuchsia boliviana. Gráfico del número de flores abiertas (A) o frutos maduros (B) de Fuchsia boliviana (Onagraceae) a lo largo de la duración de este estudio. La fecha de la primera visita fue el 15 de septiembre, y la última fue el 22 de diciembre. Graficamos plantas regadas (triángulos) y no regadas (círculos).
Quantification of herbivory
The sampling period corresponded to the peak of insect herbivores activity in the Yungas (Monmany and Aide 2009). During fruiting (October), we randomly collected five leaves from each of the 20 plants included in the study (a total of 100 leaves). One of us would close the eyes and bring the hand with one stretched finger closer to the plant; the first leaf touched was the one we collected. In order to choose the newer leaves, the finger was kept around the tip of the branches, and not at their bases. The leaves were taken to the lab to quantify herbivory. Each leaf was set flat on a white sheet of paper next to a graduated ruler for scaling, and photographed with a camera of 8-megapixel resolution using flash and a white artificial ambient light. We quantified removed leaf area, therefore considering only herbivory by chewing insects such as Lepidoptera larvae, Orthoptera, Mollusca and Coleoptera.
We analyzed the photographs of leaves using the software ImageJ version 1.5i (Schneider et al. 2012). Before taking measurements for each leaf we calibrated the pixel scale in ImageJ following a 1.5 cm scale included in each picture. We then converted each image to an 8-bit format (grayscale) to later transform it to a binary black-and-white scale (BnW) in order to choose the exact leaf contour to measure the removed area. To estimate the original leaf area (cm2) for each leaf we hand-drew a polygon following the leaf edge using the mouse pointer. To estimate leaf area removed (LAR) we calculated the difference between the original leaf area and the remaining leaf area, which we obtained with the wand tracing tool. In order to correct the effect of the leaf area we calculated the proportion of removed leaf area, with the formula pLAR=LAR/ original leaf area.
Data analysis
We first plotted the number of flowers and the number of fruits from the beginning of the study in September to the end, in December, to visually explore the phenology of F. boliviana. To test whether an increment in water affected flowering, fruiting or herbivory in this species, we restricted the flowers and fruit data sets based on plant phenology. Data included in the analysis of flowers went between October 7, when fruits started to open, and November 11, when almost no flowers remained on the plant. Analyses of fruit data went from November 15, when fruits started to ripen, until the end of the study, December 22, when almost no fruits remained on the plants. We performed three separate paired Wilcoxon signed-rank tests to compare the number of flowers, the number of fruits and pLAR between watered and unwatered plants. To see the effect of watering on the number of flowers, we calculated the mean number of flowers per plant across the complete flowering season, and compared that between plants in the watered and unwatered treatments. We did the same for fruits.
RESULTS
Fuchsia boliviana started flowering around October 7 and the last flowers wilted and fell off the plant just after November 11 (Figure 2). Fruits started to develop on the plants around November 2, and started ripening around November 15 (Figure 2). By December 22, most fruits had been consumed, had rotten, or fallen off the plant. As can be seen in (Figure 2), the overall flowering and fruiting patterns between watered and unwatered plants were similar.
The mean number of flowers for unwatered plants was 13.13±9.53 (mean±SD) while for watered plants, it was 14.99±14.37. The number of flowers did not differ significantly between treatments (V=26, P=0.73) (Figure 3). Mean number of fruits for unwatered plants was 9.26±10.11, while for watered plants it was 9.28±10.56. The mean number of fruits did not differ significantly between treatments (V=21, P=0.91) (Figure 3).
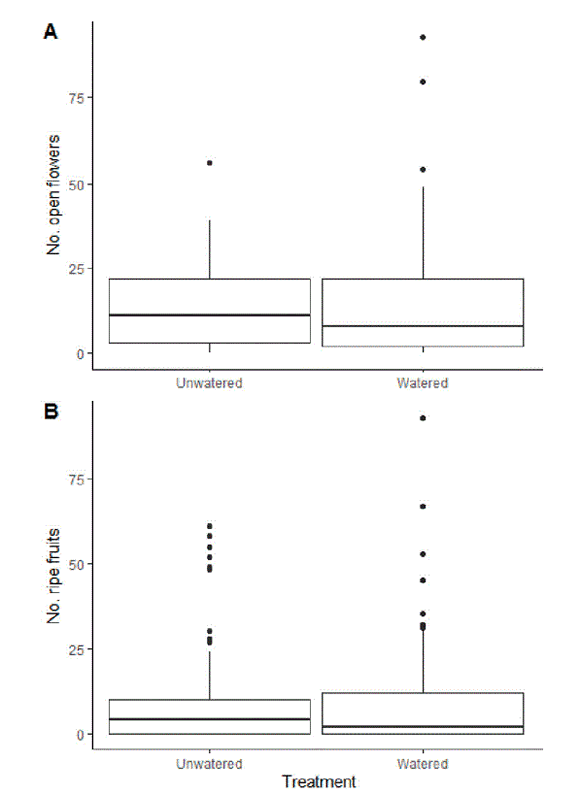
Figure 3. Boxplot of the total number of open flowers (A) and fruits (B) in unwatered versus watered Fuchsia bolivana (Onagraceae) plants. The lower hinge corresponds to the 25th percentile and the upper hinge, to the 75th percentile. The whiskers extend from the hinges to the largest or smallest values no further than 1.5 times the inter-quartile range (IQR), which represents the distance between the first and third quartiles. Data beyond the end of the whiskers are considered outlier points and are plotted individually.
Figura 3. Boxplot del número total de flores abiertas (A) y frutos (B) en plantas regadas y no regadas de Fuchsia boliviana. La caja representa los datos entre los percentiles 25 y 75. Los bigotes se extienden hasta los valores más bajos y más altos, con un límite de 1.5 veces el rango entre cuartiles, que representa la distancia entre el primer y tercer cuartiles. Los datos que están fuera del extremo de los bigotes se consideran atípicos y se grafican.
The median percentage of leaf area removed from unwatered plants was 0.04%, while from watered plants, it was 0.03%. No significant difference was found in herbivory between treatments either (V=23, P=0.70) (Figure 4).

Figure 4. Boxplot of the percentage of removed leaf area (pRLA) between unwatered and watered Fuchsia bolivana (Onagraceae) in plants. The lower hinge corresponds to the 25th percentile, and the upper hinge, to the 75th percentile. The whiskers extend from the hinges to the largest or smallest values no further than 1.5 times the inter-quartile range (IQR), which represents the distance between the first and third quartiles. Data beyond the end of the whiskers are considered outlier points and are plotted individually.
Figura 4. Boxplot del porcentaje de área foliar removida en plantas regadas y no regadas de Fuchsia boliviana. La caja representa los datos entre los percentiles 25 y 75. Los bigotes se extienden hasta los valores más bajos y más altos, con un límite de 1.5 veces el rango entre cuartiles, que representa la distancia entre el primero y tercer cuartiles. Los datos que están fuera del extremo de los bigotes se consideran atípicos y son graficados individualmente.
DISCUSSION
We described Fuchsia boliviana's phenology in its austral distribution and found that this species was not affected by the water increment to which we subjected the study plants: the number of flowers, the number of fruits and the amount of herbivory was similar between watered and unwatered plants. Yet our results on phenology showed a slight trend for watered plants to start flowering later and, perhaps, with a bit more intensity than unwatered plants. This suggests that water increment may have an effect on the timing of flowering. Fruit production, though, did not show that trend. Yet this should be explored in more depth before drawing further conclusions.
Five different options may explain why water increment did not affect the fitness of F. boliviana. First, watered plants of this mesophytic species may be allocating resources in organs different than reproductive ones, such as overall leaf mass (i.e., total leaf area, total number of leaves), as has been shown for other Fuchsia sp. (Pagter and Petersen 2008). In addition, previous studies on the relationship between water and the development of organs such as roots have shown a high variability within Onagraceae (Hussner 2010) and even within Fuchsia sp. (Berry et al. 1988; Graves and Zhang 1996; Caranqui Aldaz 2011), and this relationship is apparently dependent on habitat seasonality. Because we did not consider these response variables, we may have missed this aspect of water increment on productivity and suggest that a more complete assessment would be achieved if leaf mass is included, as this may be an indicator of root mass (Pagter and Petersen 2008). Second, perhaps we should have started our treatment earlier in the dry season, as a change in water increment might have a greater impact when that resource is most limiting (Lance et al. 2017). Spring rains started just a couple of weeks after we started the experiment. It is possible that the plants had already allocated resources to flower production before our experiment started, as the species is adapted to this region's climate patterns and may respond to other triggers, such as light regime changes (Parra-Tabla and Bullock 2000; Brock et al. 2017). Further studies should explore whether watering the plants earlier in the dry season makes more of a difference in the fitness of plants in seasonal environments such as our study site. Third, it is possible that the way we watered the plants was inappropriate to make a difference in plant fitness. Pouring the water all at once might have caused some of the water to drain away on the surface before being absorbed by the soil. Fourth, plants may be accessing underground water and, hence, the reproductive process of the plant may be completely independent of our experimental water addition. However, we included very small plants (just a little over 1 m in height and a diameter at the base of the trunk of less than two centimeters), for which we would not expect deep roots. Yet this remains as a speculation until we learn more about the root characteristics of F. boliviana. Finally, herbivory was quantified only at the end of our study. Though our method tended to choose only leaves that were new in the season and had not been damaged before our experiment started (Lomáscolo and Monmany-Garzia, personal observation), quantifying herbivory also at the starting time of the experiment would have helped examine temporal responses to herbivory (Sanders-Demott et al. 2018). Perhaps marking young leaves as plants were starting to produce them at the beginning of the growing season, and then quantifying herbivory on those leaves would have been the most appropriate method.
Water was not a determinant for productivity of F. boliviana in our study. Previous studies showed that water availability is especially limiting for plants in dry climates (Dudley et al. 2018), in contrast to our study site, where more humid conditions may soften the responses of F. boliviana. Longer studies encompassing more than one flowering/fruiting season could also help to determine if there might be a delayed response to the water added during the previous reproductive season.
ACKNOWLEDGEMENTS
This study was part of the training of two undergraduate students in the program for Biology students, PECARIES (Training Program for Undergraduate Students in Ecological Research in the Subtropics) by the Institute of Regional Ecology (IER, CONICET-National University of Tucumán). The students are coauthors of this manuscript, PS and FA. We thank the mentioned program for resources supporting this study. The San Javier Park administration gave permissions to conduct this study. We thank JLT for help with fieldwork and Pedro Blendinger for the picture of Fuchsia fruits in Figure 1.
REFERENCIAS
1. Aldaz, J. C., and H. Politécnico. 2011. Avances taxonómicos y de propagación del género Fuchsia en Ecuador. Q'EUÑA 4:39-45. https://doi.org/10.1002/j.1537-2197.1986.tb10902.x. [ Links ]
2. Averett, J. E., W. J. Hahn, P. E. Berry, and P. H. Raven. 1986. Flavonoids and flavonoid evolutino in Fuchsia (Onagraceae). American Journal of Botany 73:1525-1534. [ Links ]
3. Bernacchi, C. J., and A. Vanloocke. 2015. Terrestrial Ecosystems in a Changing Environment: A Dominant Role for Water. Annual Review in Plant Biology 66:599-622. https://doi.org/10.1146/annurev-arplant-043014-114834. [ Links ]
4. Berry, E., B. A. Stein, N. L. Street, and J. Nwicke. 1988. Fuchsia pachyrrhiza (Onagraceae), a Tuberous New Species and Section of Fuchsia from Western Peru. Systematic Botany 13:483-492. https://doi.org/10.2307/2419193. [ Links ]
5. Berry, P. E. 1982. The Systematics and Evolution of Fuchsia Sect. Fuchsia (Onagraceae). Annals of the Missouri Botanical Garden. Missouri Botanical Garden 69:1-198. https://doi.org/10.2307/2398789. [ Links ]
6. Berry, P. E., W. J. Hahn, K. J. Sytsma, J. C. Hall, and A. Mast. 2004. Phylogenetic relationships and biogeography of Fuchsia (Onagraceae) based on noncoding nuclear and chloroplast DNA data. American Journal of Botany 91:601-614. https://doi.org/10.3732/ajb.91.4.601. [ Links ]
7. Breedlove, D. E., P. E. Berry, and P. H. Raven. 1982. The Mexican and Central American Species of Fuchsia (Onagraceae) Except for Sect. Encliandra. Annals of the Missouri Botanical Garden 69:209-234. https://doi.org/10.2307/2398791. [ Links ]
8. Brock, M. T., R. L. Winkelman, M. J. Rubin, C. E. Edwards, B. E. Ewers, and C. Weinig. 2017. Allocation to male vs female floral function varies by currency and responds differentially to density and moisture stress. Heredity 119:349-359. https://doi.org/10.1038/hdy.2017.41. [ Links ]
9. Cabrera, A. L., and A. Willink. 1980. Biogeografía de América Latina. Secretaría General de la Organización de los Estados Americanos, Programa Regional de Desarrollo Científico y Tecnológico. 2da ed. Pp. 122. [ Links ]
10. Caranqui Aldaz, J. 2011. Avances taxonómicos y de propagación del género Fuchsia en Ecuador - Taxonomic and propagation advances of the genus Fuchsia in Ecuador. Q'EUÑA 4:39-45. [ Links ]
11. Carlquist, S. 1975. Wood anatomy of Onagraceae, with notes on alternative modes of photosynthate movement in dicotyledon woods. Annals of the Missouri Botanical Garden 62:386-424. https://doi.org/10.2307/2395205. [ Links ]
12. Carroll, A. B., S. G. Pallardy, and C. Galen. 2001. Drought stress, plant water status, and floral trais expression in fireweed, Epilobium angustifolium (Onagraceae). American Journal of Botany 88:438-446. https://doi.org/10.2307/2657108. [ Links ]
13. Case, A. L., and T. -L. Ashman. 2005. Sex-specific Physiology and its Implications for the Cost of Reproduction. Pp. 126-154 in E. G. Reekie and F. A. Bazzaz (eds.). Reproductive allocation in plants. Elsevier Academic Press, London, UK. https://doi.org/10.1016/B978-012088386-8/50005-3. [ Links ]
14. Chang-Yang, C. -H., I. -F. Sun, C. Tsai, C. Lu, and C. Hsieh. 2016. ENSO and frost codetermine decade-long temporal variation in fl ower and seed production in a subtropical rain forest. Journal of Ecology 104:44-54. https://doi.org/10.1111/1365-2745.12481. [ Links ]
15. Dudley, L. S., M. T. K. Arroyo, and M. P. Fernández-Murillo. 2018. Physiological and fitness response of flowers to temperature and water augmentation in a high Andean geophyte. Environmental and Experimental Botany 150:1-8. https://doi.org/10.1016/j.envexpbot.2018.02.015. [ Links ]
16. Eckhart, V. M., I. Singh, A. Louthan, A. Keledjian, A. Chu, D. Moeller, and M. Geber. 2010. Plant Soil Water Relations and Species Border of Clarkia xantiana ssp. xantiana (Onagraceae). International Journal of Plant Sciences 171:749-760. https://doi.org/10.1086/654845. [ Links ]
17. Galen, C. 2005. It never rains, but then it pours. In E. Reekie and F. Bazzaz (eds.). Reproductive allocation in plants. Elsevier Academic Press, London, UK. https://doi.org/10.1016/B978-012088386-8/50003-X. [ Links ]
18. Giannini, N. P. 1999. La interacción de aves-murciélagos-plantas en el sistema de frugivoría y dispersión de semillas en San Javier, Tucumán, Argentina. Universidad Nacional de Tucumán. [ Links ]
19. Grau, H. R. 2002. Scale-dependent relationships between treefalls and species richness in a neotropical montane forest. Ecology 83:2591-2601. https://doi.org/10.1890/0012-9658(2002)083[2591:SDRBTA]2.0.CO;2. [ Links ]
20. Graves, W. R., and H. Zhang. 1996. Relative water content and rooting of subirrigated stem cuttings in four environments without mist. Horticultural Science 31:866-868. https://doi.org/10.21273/HORTSCI.31.5.866. [ Links ]
21. Hahn, P. G., and J. L. Maron. 2018. Plant water stress and previous herbivore damage affect insect performance. Ecological Entomology 43:47-54. https://doi.org/10.1111/een.12468. [ Links ]
22. Hunzinger, H. 1997. Hydrology of Montane Forests in the Sierra de San Javier, Tucuman, Argentina. Mountain research and development 17:299-308. https://doi.org/10.2307/3674020. [ Links ]
23. Hussner, A. 2010. Growth response and root system development of the invasive Ludwigia grandiflora and Ludwigia peploides to nutrient availability and water level. Fundamental and Applied Limnology / Archiv für Hydrobiologie 177:189-196. https://doi.org/10.1127/1863-9135/2010/0177-0189. [ Links ]
24. Lambrecht, S. C. 2013. Floral water costs and size variation in the highly selfing Leptosiphon bicolor (Polemoniaceae). International Journal of Plant Sciences 174:74-84. https://doi.org/10.1086/668230. [ Links ]
25. Lance, R. F., P. Bailey, D. L. Lindsay, and N. S. Cobb. 2017. Precipitation and the robustness of a plant and flower-visiting insect network in a xeric ecosystem. Journal of Arid Environments 144:48-59. https://doi.org/10.1016/j.jaridenv.2017.03.015. [ Links ]
26. Leathwick, J. R., and D. Whitehead. 2001. Soil and atmospheric water deficits and the distribution of New Zealand's indigenous tree species. Functional Ecology 15:233-242. https://doi.org/10.1046/j.1365-2435.2001.00504.x. [ Links ]
27. Malizia, A., T. A. Easdale, and H. R. Grau. 2013. Rapid structural and compositional change in an old-growth subtropical forest: using plant traits to identify probable drivers. PloS One 8:e73546. [ Links ]
28. Malizia, A., and H. R. Grau. 2006. Liana-host tree associations in a subtropical montane forest of north-western Argentina. Journal of Tropical Ecology 22:331-339. https://doi.org/10.1371/journal.pone.0073546. [ Links ]
29. Monmany, A. C., and T. M. Aide. 2009. Landscape and community drivers of herbivore parasitism in Northwest Argentina. Agriculture, Ecosystem and Environment 134:148-152. https://doi.org/10.1016/j.agee.2009.06.013. [ Links ]
30. Nafus, M. G., T. D. Tuberville, K. A. Buhlmann, and B. D. Todd. 2017. Precipitation quantity and timing affect native plant production and growth of a key herbivore, the desert tortoise, in the Mojave Desert. Climate Change Responses 4:4. https://doi.org/10.1186/s40665-017-0032-9. [ Links ]
31. Pagter, M., and K. Petersen. 2008. Drought adaptation in Fuchsia magellanica and its effect on freezing tolerance. Journal of the American Society for Horticultural Science 133:11-19. https://doi.org/10.21273/JASHS.133.1.11. [ Links ]
32. Parra-Tabla, V., and S. H. Bullock. 2000. Phenotypic natural selection on flower biomass allocation in the tropical tree Ipomoea wolcottiana Rose (Convolvulaceae). Plant Systematics and Evolution 221:167-177. https://doi.org/10.1007/BF01089292. [ Links ]
33. Pezzola, E., S. Mancuso, and R. Karban. 2017. Precipitation affects plant communication and defense. Ecology 98:1693. https://doi.org/10.1002/ecy.1846. [ Links ]
34. Puchulu, M. E., and D. S. Fernández. 2014. Características y distribución espacial de los suelos de la provincia de Tucumán. Pp. 1-17 in S. Moyano, M. E. Puchulu, D. Fernandez, G. Aceñolaza, M. E. Vides and S. Nieva (eds.). Geología de Tucumán. Colegio de Geólogos de Tucumán, Tucumán, Argentina. [ Links ]
35. Ripoll, J., L. Urban, M. Staudt, F. López-Lauri, L. P. R. Bidel, and N. Bertin. 2014. Water shortage and quality of fleshy fruits-making the most of the unavoidable. Journal of Experimental Botany 65:4097-4117. https://doi.org/10.1093/jxb/eru197. [ Links ]
36. Sanders-Demott, R., R. McNellis, M. Jabouri, and P. H. Templer. 2018. Snow depth, soil temperature and plant-herbivore interactions mediate plant response to climate change. Journal of Ecology 106:1508-1519. https://doi.org/10.1111/1365-2745.12912. [ Links ]
37. Schneider, C. A., W. S. Rasband, and K. W. Eliceiri. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:617-675. https://doi.org/10.1038/nmeth.2089. [ Links ]
38. Zanor, M. I., S. Osorio, A. Nunes-Nesi, M. Carrari, F. Lohse, B. Usadel, C. Kuhn, W. Bleiss, P. Giavalisco, L. Willmitzer, R. Sulpice, Y. -H. Zhou, and A. R. Fernie. 2009. RNA interference of LIN5 in tomato confirms its role in controlling brix content, uncovers the influence of sugars on the levels of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiology 150:1204-1218. https://doi.org/10.1104/pp.109.136598. [ Links ]