Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista de Ciencia y Tecnología
versão On-line ISSN 1851-7587
Rev. cienc. tecnol. no.23 Posadas jun. 2015
ÁREA: INGENIERÍA Y TECNOLOGÍA
Evaluation mass transfer during the drying of watermelon rind to obtain and characterize its flour
Evaluación de la transferencia de masa durante el secado de la corteza de la sandía para la obtención y caracterización de la harina
Julianne V. F. Portela1 *, Thayze R. B. Pessoa2, Jacinete Pereira Lima2, Ânoar A. El-Aouar2
1 Universidade Federal de Piauí; Departamento de Nutrición; Campus Senador Helvídio Nunes de Barros; Calle Duarte Cícero, 905, Barrio Rush, Código postal: 64607-670, Picos-PI; Teléfono: (+55 89) 3422-1018
2 Universidad Federal de Paraíba, Departamento de Tecnología, Química y de Alimentos, Barrio: Cidade Universitária, Campus Universitário I; Código postal: 58059-900, João Pessoa-PB
* E-mail: julianneportela@ufpi.edu.br
Abstract
The purpose of this study was to evaluate the convective drying process of the watermelon rind and develop flour with technological and nutritional application to human nutrition. The rind was cut longitudinally (5 mm thick), preserving the epicarp and mesocarp, and it was subjected to convective drying under air temperature conditions (40-60 ºC) and with the air speed fixed (1.0m.s-1). Studies of the drying kinetics, mathematical modeling (Fick, Page and Two Parameter Empirical) of the experimental curves and determination of the effective diffusivity, water and moisture activity were conducted as parameters to define the best technological process conditions. Physiochemical characteristics of the watermelon rind flour were determined. The temperature increase resulted in the increase of the water effective diffusivity values, having the Page and Two Parameter Empirical models as those that were better adjusted to the experimental data. Based on the quality responses, 60 ºC was defined as the best condition, resulting in flour with expressive protein content and minerals.
Keywords: Citrullus lanatus; Effective diffusivity; Protein; Mineral salts; Bread making.
Resumen
El objetivo fue evaluar el proceso de secado por convección de la corteza de la sandía, y el desarrollo de la harina con aplicación en la nutrición humana. La materia prima se cortó longitudinalmente (5 mm de espesor), preservando el epicarpio y mesocarpio y fue sometida a secado por convección en diferentes condiciones de temperatura del aire (40-60 ºC) y una velocidad fija del aire (1.0 m.s-1). Se llevó a cabo el estudio de la cinética de secado, el ajuste a los modelos matemáticos (Fick, Page y de dos parámetros empíricos),la determinación de las curvas experimentales y de la difusividad efectiva, la actividad del agua y la humedad, como parámetros para definir la mejor condición del proceso tecnológico. Se determinó las características fisicoquímicas de la harina de cáscaras de sandía. El aumento de la temperatura produjo un aumento en los valores de difusividad efectiva de agua y el modelo empírico de los dos parámetros fue el que mejor ajustó a los datos experimentales. La temperatura de 60 ºC se definió como la mejor condición del secado, lo que resulta en la harina con cantidades significativas de proteínas y minerales.
Palabras clave: Citrullus lanatus; Difusividad efectiva; Proteínas; Minerales; Hornear.
Introduction
Watermelon (Citrullus lanatus) is an important cucurbit crop, accounting for 7% of the worldwide area devoted to vegetable production. The annual world production of watermelon is about 90 million tons, making it among the top five most consumed fresh fruits (1). This high production, in the other hand, could generate an expressive increase in the agroindustrial residues due to inadequate conducts mainly that from mechanical injury during transport, favouring the physical damage and depreciate the watermelon quality characteristics.
This makes it relevant to increment proposals for the rind vegetable processing, applying technologies relatively cheap and with an easy accessibility to the agroindustrial sector, as the convective drying. It is defined as an operation in which heat is supplied to a determined wet material to vaporize certain water content from this material, thus obtaining a solid dried product. It is a simultaneous heat and mass transfer process, accompanied by phase change and increment shelf-life (2; 3; 4).
It is essentialy a nutrient concentration process, it can also contribute to the reduction of the negatives impacts to the environment and in the income generation to the producers of this vegetable.
Thus, the present study aimed to determine the characteristics of the convective drying process of watermelon rind, considering jointly the epicarpand mesocarp, evaluate the predictive ability of mathematical models to obtain effective diffusivity of water under the process conditions, and obtain and characterize a flour product from the dried watermelon rind.
Materials and Methods
Watermelon (Citrullus lanatus cv 'Crimsom Sweet') were purchased in a local market (EMPASA, João Pessoa city, Brazil), were selected according to two steps, in order to assure homogeneity and final product, as well as stalk color (greenish), rejecting the samples that presented physical injuries. The material was obtained on the day of each experiment and transported to the Unit Operations Laboratory of the Universidade Federal da Paraíba - Campus João Pessoa.
The rind was sliced longitudinally with a thickness of 5mm, maintaining the epicarp and mesocarp, this thickness being measured and standardized patterned with the aid of calipers (Norfol Ivonica®). The following the rinds were analyzed in triplicate: water activity (Aqualab®, Model CX-2), moisture content, soluble solids, acidity, pH, reducing sugars, total sugar and starch (5).
A continuous flow fixed bed dryer was used and the tests were conducted at three different temperatures (40, 50 and 60 °C) with a constant air velocity (1.0 m.s-1). The dryer system consisted of vertical air flow through trays and was arranged as a closed circuit. For the air heating, three electric resistances were used (one of 500 W, one of 1000 W and one of 1500 W), which could be worked independently. A thermo-hygrometer (Alla France®) was used in order to measure the dry bulb temperature, as well as the drying air humidity. A digital anemometer (Velocicheck -TSI®) was used to measure the drying air velocity.
Maintaining these conditions, the samples were placed in second dryer tray (from bottom to top). Thus, around 17.00 ± 0.02 g of material, previously weighed on a semi-analytical scale being layed out in stainless steel baskets.
Samples moisture content, during the air-drying process, was gravimetrically determined from the samples initial moisture content (before air-drying process). Samples weights were measured using a semi-analytical balance with precision of 0.01 g. Weighting intervals of 15 min were used during the first hour of processing, 30 min for the next 2 h, and then 1 h until the dynamic equilibrium between the sample moisture content and drying air humidity was reached when the sample weight became constant (maximum variation of 0.05 g). The experiments were performed in duplicate.
At the end of each experiment, samples were subjected to a vacuum oven (70 °C/24 hours) to determine the dry weight, which was used for calculating the drying kinetics, whereas another part was subjected to the grinding processinan industrial micro process or for 2 minutes, to obtain flour with uniform particle size.
The drying kinetics was studied through adimensional moisture curves in function of process time (Equation 1) as well as the dry ingrate curves (dX/dt) which were calculated by the moisture content derivative with respect to time (Equation 2).
![]()
in which: RX = ratio of moisture content, adimensional, X(t) = moisture content of the material at a given drying time; Xo = moisture content of material at the start of the process; Xe = moisture content of the material at the moment of dynamic equilibrium.
The mathematical modeling of the drying kinetics experimental data was also performed using the diffusion model (Fick's 2 nd Law) (Equation 3) (6; 7), considering that sample has infinite slab geometry; empirical models is Page's equation (Equation 4) (8) and empirical model is the two term exponential model (Equation 5).
![]()
In which: t =time (minutes), n = number of readings; dX/dt= drying rate (kgH2O/kgms.min); Xn and Xn-1 = moisture (kgH2O/kgms) at time "n" and time "n-1".
The mathematical modeling of the drying kinetics experimental data was also performed using the diffusion model (Fick's 2nd Law) (Equation 3) (6; 7), considering that sample has infinite slab geometry;empirical models is Page's equation (Equation 4) (8) and empirical model is the two term exponential model (Equation 5).
![]()
where: (X(t) - Xe)/( Xo - Xe) = ratio of moisture on dry basis(adimensional); X(t) = average moisture content at instant t (gw/gdm); Xe= equilibrium moisture content (gw/ gdm); Xo= the initial moisture content (gw/gdm, kg water/kg dry mass); Def= effective diffusivity of water (m2.s-1);L= the characteristic length, sample half-thickness (m), t= the drying time (s).
![]()
where: K is the drying constant; b is the Page's parameter and t is the drying time.
![]()
where: A and B are the parameters and t is the drying time.
For the adjustment of the experimental data to mathematical drying models, the determination coefficient 2 Rand mean relative deviation module P (Equation 6), using the computational program Statistic 7.0 (9) was conducted.
![]()
where: N is the number of experimental data, VE is the experimental value and VP is the calculated value (10).
The effective diffusivity (Def) was obtained by fitting the experimental data to Fick´s diffusional model, applying the nonlinear estimation. Def was also calculated using the empirical models by an analogy to Fick's equation, considering the same geometry.
In order to evaluate and identify the best drying condition to obtain the dried watermelon rind flour, first the assessment of the quality parameters (water activity and final moisture) result of each drying process was conducted. These analyzes were performed in triplicate (5). Comparing the results of these quality parameters to the drying kinetics analysis we obtained the best process temperature. The flour then obtained under this better condition was then physicochemically characterized, in triplicate: water activity (Aqualab®, Model CX-2), moisture content, acidity and pH, proteins, lipids, and starch, reducing sugars and total sugar, using Lane and Eynon method (5).
Results
The Table 1 show physico-chemical characterization of fresh watermelon (Citrullus lanatus) rinds and flour.
Table 1: Physico-chemical characterization of fresh watermelon (Citrullus lanatus) rinds and flour.
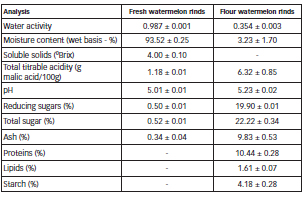
Figure 1 show the drying behavior as a function of time for the watermelon rinds for the studied drying temperatures.
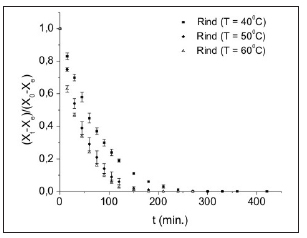
Figure 1: Dimensionless moisture content as a function of process time for the watermelon rinds under different temperatures.
Figures 2 (a) and (b) show the drying rate as a function of moisture content, detailing the structural behavior of the material in the processes at different air drying temperatures.
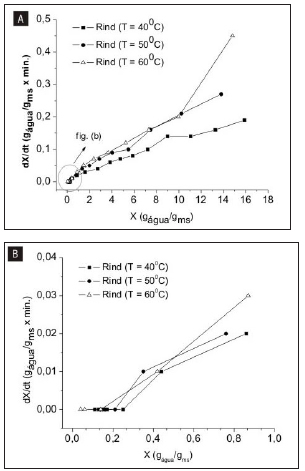
Figure 2: Drying rate as a function of samples moisture content for watermelon rind. (a) all drying rate and moisture content points; (b) moisture content less than 1.00gwater/gdry matter
Figure 3 (a, b, c) show the mathematical modelling for watermelon rind. Table 2 to 4 presents the water effective diffusivity values, the correlation coefficients (R2), the mean relative deviation module (P) and other parameters.

Figure 3: Mathematical modeling of watermelon rind drying (a), (b) and (c) at different temperatures.
Table 2: Fick's equation parameters and statistical results for watermelon rind drying at different temperatures.

Table 3: Empirical equation parameters and statistical results for watermelon rind drying at different temperatures.

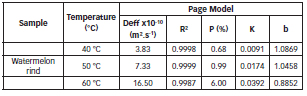
Table 4: Page equation parameters and statistical results for watermelon rind drying at different temperatures.
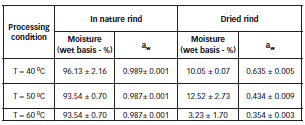
The Table 5 show moisture content and water activity of in nature and dried watermelon rind.
Table 5: Moisture content and water activity of in nature and dried watermelon rind.
Discussion
Due to the absence of studies jointly examining the epicarp and mesocarp, the results were compared with the study of the mesocarp conducted by Barroso et al. (11). Thus, it is observed (Table 1) that the pH value was close to that referenced in the literature, while the moisture content and water activity were slightly lower, especially the value for soluble solids. The acid content was much higher and the ash much lower than that found in the literature.
Considering the falling rate period, there was no significance difference between drying at 500C and 600C, showing drying curves more accentuated, with a tendence to the dinamic equilibrium after 120 min of processing. It can also be seen that the lower temperature required a higher processing time, reaching the dinamic equilibrium after 180 min (Figure 1 (a)).These data corroborate with findings by Azoubel et al. (12) when studying the drying of "Tommy Atkins" mango rind.
Confirming the non-difference between the higher temperatures, we have a similar linear behavior among these samples, implying a higher drying rate at temperatures of 50oC and 60oC compared to the drying process at 40 oC. It was observed that drying rates, occurred at falling drying rate period, were higher at the beginning of drying, when the moisture content of the samples were higher. It is noted therefore that the drying air temperature exercises a directly proportional influence on the drying rate, occurring a rapid decline on its value for the studied samples. This behavior, in relation to temperature, was similar to the results related by Azoubel et al. (12), Fiorentin et al. (13), Vilhalva et al. (14), Ferreira e Pena (4).
The biological material showed uniform drying throughout the process, namely the shrinkage of the material was proportional to the water output, therefore the rind showed little resistance to the effective diffusivity of water. It is emphasized that no difference was clearly observed in drying rates among samples when compared to the same amount of moisture near the dynamic equilibrium point (Figures 2 (b)). As observed by El-Aouar (15), Fiorentin et al. (13) and Vilhalva et al. (14).
The moisture content data at various temperatures obtained in the experiment were converted to the moisture ratio and adjusted to the three models, while the evaluation period was standardized to a 90 minute drying process.
According to Tables 2 to 4 it can be seen that the higher the process temperature, the higher the value of the effective diffusivity of water for each modelling, agreeing with the analyses of the drying rate graphs (Figure 2 (a) and (b)). It is verified that the effective diffusivity data are high for the watermelon rind, because when considering its geometric aspect, presents an extensive surface area in contact with the drying air. As observed by Vilhalva et al. (2012) (14) in evaluation cassava peel drying process.
It can be seen in the Figure 3 that the Difusional model presented low flexibility to the experimental data, this fact being justified by the reduced degrees of freedom of the referred mathematical function, that resulted in low determination coefficient values and high values for the average relative deviation (P > 10%) (Table 2).
When correlating the results of the Fick model with the data presented in Tables 3 and 4,it can be mentioned that the two parameter empirical exponential model, and the Page model resulted in R2 values nearest the unit, besides P% values within those proposed by Lomauro et al.(10) and Mohapatra e Rao (16), in other words, under 10%. The presented data infer that these last two models are those that best predict the experimental data and corroborate with that found by Karim e Hawlader (17); Vilhalva et al. (14) and Monteiro et al. (18) on studying tropical fruits.
Rizvi (19) states that the effective diffusivity of water is dependent, among other factors, on the temperature of the drying air. This fact can be observed in the study of drying watermelon rinds because when analyzing Tables 2-4 it can be seen that the temperature increase, irrespective of the mathematical model used, results in an increase in the effective diffusivity of water. The diffusivities values were in the order of 10-10 m².s-1 for all temperatures. Park et al. (20), drying fruits, found diffusivities values in the order of 10-10 m².s-1, what is in agreement with the results obtained in this study and according Costa et al.(21).
Table 5 shows that the flour of dried watermelon rind obtained under all temperature conditions showed final moisture compatible with that recommended by Resolution No.263 of 2005, which indicates that this type of product should provide a maximum moisture of 15% (22).
The in nature watermelon rind presents a high level of water activity which promotes the growth of bacteria, yeast and mold, and is therefore classified as perishable. After the drying processes at 50 oC and 60 oC the water activity values reached make these products microbiologically stable, because these values presented are below the minimum necessary for the development of major pathogens (bacteria, yeasts and molds) (23).These values were consistent with the increase in process temperature, because, as shown in Figure1,the higher the temperature the faster the drying process, reaching a lower equilibrium moisture content. Comparing the results of these quality parameters to the drying kinetics analysis, the 60 oC temperature is suggest ed as the most adequate to obtain dried watermelon rind flour.
The protein and lipid content of the flour of the dried watermelon rind were equivalent to that found in the flour of the passion fruit rind; while the ash content was higher (24), presenting an increase of 391.50%. That high ash content leads to a high concentration of minerals, suggesting that it is a mineral source product.
According to Resolution Nº 54 (25) a solid food is considered a proteins source when it contains at least 10% of the Recommended Daily Intake (RDI indicated in Resolution Nº 269 of September 22, 2005 (26), for different groups of individuals. Based on the legislation, the flour of the dried watermelon rind can be considered a protein source for all groups of individuals, pregnant and lactating women, the group with highest need of this nutrient. According to the same Resolution Nº 54 (25), a food is considered a product of low of total fat content when it presents a maximum of 3 grams of fat per 100 grams of product. As such, all of the analyzed products fit in this category. It is worth pointing out that the developed flour presented low moisture and water activity levels. These contents are characteristic of highly hygroscopic products, in other words, those with high rehydration capacity. Studies should therefore begin on the analysis of shelf life associated to the sorption isotherm.
Other relevant characteristic for this product originating from the watermelon rind was the perception of a bitter flavor originating from a terpenoid substance known as cucurbitacina, positively very important for presenting a wide spectrum of biological activity, such as anti-inflammatory and antitumor properities. The obtained flour promotes the useful life increase of a highly perishable raw material, and may be inserted in the food industry as source of mineral and protein enrichment in food products, mainly of the bakery sector.
Conclusions
The convective drying of watermelon rind is best predicted by the two-parameter empirical exponential and Page models. The whole drying process occurs at a decreasing rate, resulting in an effective diffusivity of water on the order of 10-10m2.s-1. The temperature of 60 oC is the best drying condition for providing the final product with a microbiological stability classification.
The flour of dried watermelon rind has characteristics indicative of being a good source of protein and minerals that favor its application in the food industry.
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) the funding indispensable for the completion of this work.
1. FAO - Food and Agriculture Organization of the United Nations. FAOSTAT statistic database (2012). Available <http://www.faostat.fao.org/>. Accessed on 09 April 2014. [ Links ]
2. Ribas, A. I.; Cánovas, G. V. B.; Garza, S. G.; Añó, V. G. Métodos experimentales en la ingeniería alimentaria. Zaragoza: Acribia, 2000. 292 p. [ Links ]
3. Lewicki, P. P.; Jakubczyk, E. Effect of hot air temperature on mechanical properties of dried apples. Journal of Food Engineering, v. 64, nº 3, p. 307-314. 2004. [ Links ]
4. Ferreira, M. F. P.; Pena, R. S. Estudo da secagem da casca do maracujá amarelo. Revista Brasileira de Produtos Agroindustriais, Campina Grande, v. 12, nº 1, p. 15- 28, 2010. [ Links ]
5. AOAC. Official methods of analysis of the Association of Analytical Chemists International. (18th). USA: Washington. 2005. [ Links ]
6. Brooker, D.B.; Bakker-Arkema, F.W.; Hall, C.W. Drying Cereal/ Grains. Connecticut: The AVI Publishing Company, 1974. 265 p. [ Links ]
7. Cranck, J. The mathematics of diffusivity (2nd ed). Oxford: Clarendon Press. 1975. [ Links ]
8. Page, G.E. Factors influencing the maximum of air drying shelled corn in thin layer. (1949). Thesis (M. Sc.) - Purdue University, Indiana, 1949. [ Links ]
9. STATSOFT. Statistica for windows, Tulsa, USA, 2004. [ Links ]
10. Lomauro, C. J.; Bakshi, A. S.; Labusa, T. P. Evaluation of food moisture sorption isotherm equations. Part I: fruit, vegetable and meat products. Lebensmittel - Wissenschaft and Technologies, v. 18, p. 112-122. 1985. [ Links ]
11. Barroso, A. P. S.; Macedo, A. N.; Santana, J. N. S.; Silva, R. P. S.; Silva, I. R. A. Caracterização físico-química do mesocarpo da melancia (Citrullus lanatus) cultivada no Vale do São Francisco. In: III Congresso de Pesquisa e Inovação da Rede Norte Nordeste de Educação Tecnológica. Anais.Fortaleza - Ceará, 2008. [ Links ]
12. Azoubel, P. M.; Evangelista, E. C. D. A.; Oliveira, S.B.; Silva, Í. R. A.; Araújo, A. J. B. Cinética de secagem da casca de manga "Tommy Atkins". In: XVII Congresso Brasileiro de Engenharia Química, IV Congresso Brasileiro de Termodinâmica aplicada. Anais.Recife: Universidade Federal de Pernambuco, 2008. p. 1-5. [ Links ]
13. Fiorentin, L. D.; Menon, B. T.; Alves, J. A.; Tereza, S.; de Barros, D.; Pereira, N. C.; Módenes, A.N. Determinação da cinética e das isotermas de secagem do bagaço da laranja. Acta Scientiarum: Technology. Apr-Jun2010, Vol. 32 Issue 2, p. 147-152. [ Links ]
14. Vilhalva,D. A. A.; Soares Júnior, M. S.; Caliari, M.; Silva, F. A. Secagem convencional de casca demandioca proveniente de resíduos de indústria de amido. Pesq. Agropec. Trop., Goiânia, v. 42, nº 3, p. 331-339, jul./set. 2012. [ Links ]
15. El- Auoar, A. A. Estudo do processo de secagem de mamão Formosa (Carica papaya L.) fresco e pré-tratado osmoticamente. 2005. 215 f. Tese (Doutorado em Engenharia de Alimentos) - Faculdade de Engenharia de Alimentos, Universidade Estadual de Campinas, Campinas, 2005. [ Links ]
16. Mohapatra, D.; Rao, P. S. A thin layer drying model of parboiled wheat. Journal of Food Engineering, v.66, p. 513-518, 2005. [ Links ]
17. Karim, M. A.; Hawlader, M.N.A. Mathematical modelling and experimental investigation of tropical fruits drying. International Journal of Heat and Mass Transfer, v. 48, nº 23-24, p. 4914-4925, 2005. [ Links ]
18. Monteiro, L. B.; Mendonça, M.R.; Andrade, A.T.; Camargo, T.; Sousa, K.M.; Oi, R.; Moraes, M.S.; Lia, L.R.B.; Moraes Junior, D. Curva de secagem do mesocarpo do maracujá amarelo. Revista Ceciliana, Santos, v. 2, nº 1, p. 42-44, 2010. [ Links ]
19. Rizvi, S.S.H. Thermodynamic properties of food in dehydration. In: Rao, M.A.; Rizvi, S.S.H. Engineering properties of food. v. 4.New York: Marcel Dekker. p. 113-24. 1986. [ Links ]
20. Park, K. J.; Bin, A.; Brod, F. P. R.; Park, T. H. K. B. Osmotic dehydration kinetics of pear D'anjou (Pyrus communis L.). Journal of Food Engineering, v. 52, nº 3, p. 293-298, maio, 2002. [ Links ]
21. Costa, L. M.; Resende, O.; Sousa, K.A.; Gonçalves, D. N. Coeficiente de difusão efetivo e modelagem matemática da secagem de sementes de crambe . Revista Brasileira de Engenharia Agrícola e Ambiental. v. 15, nº 10, p. 1089-1096, 2011. [ Links ]
22. BRASIL. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Comissão Nacional de Normas e Padrões para Alimentos. Resolução nº 263, de 2005. Regulamento Técnico para Produtos de Cereais, Amidos, Farinhas e Farelos. Diário Oficial da União, Brasília, DF, 22 de Setembro de 2005. [ Links ]
23. ICMSF - International Commission on Microbiological Specifications for Foods. Microorganismos de los alimentos: ecologia microbiana de los productos alimentarios. [ Links ]
24. Souza, M. W. S.; Ferreira, T. B. O.; Vieira, I. F. R. Composição centesimal e propriedades funcionais tecnológicas da farinha da casca do maracujá.Alimentação e Nutrição, v. 19, nº 1, p. 33-36, jan/mar, 2008. [ Links ]
25. Brasil. Ministério da Saúde. Agência de Vigilância Sanitária. Resolução nº 54 de 12 de novembro de 2012. Regulamento sobre Informação Nutricional Complementar. Diário Oficial da União, Brasília, DF, 12 de novembro de 2005. [ Links ]
26. Brasil. Ministério da Saúde. Agência de Vigilância Sanitária. Resolução nº 269 de 22 de setembro de 2005. Regulamento técnico sobre a ingestão diária recomendada (IDR) de proteína, vitaminas e minerais. Diário Oficial da União, Brasília, DF, 22 de setembro de 2005. [ Links ]
Recibido: 17/04/2014.
Aprobado: 08/09/2014.














